Introduction
Benzodiazepines (BZDs) are commonly used psychotropic drugs to treat insomnia and anxiety 1. Their long-term use is associated with considerable adverse effects 2, such as an increased number of falls and bone fractures 2-4, a higher number of road accidents 2,5, and it has a possible role in inducing suicide 6-8. Numerous studies have shown that BZDs are still overprescribed and commonly used long-term 9,10, despite the existence of clinical guidance advising the use of non-pharmacological psychological treatments in the first-line and restricting BZD prescription to a maximum of 8-12 weeks 11. Also, BZDs are often wrongly chosen to treat anxiety disorder symptoms at the expense of adequate pharmacological treatment with antidepressants 12,13.
The consistently high prescription of BZDs in Portugal has been referenced in national and international publications over the last 20 years 14-16, with this fact being recognised as a sign of inadequate management of mental disorders and considered a serious public health concern 17-19. Moreover, The Portuguese National Health Plan issued for 2012-2016 defined specific indicators to monitor the outpatient consumption of antidepressants and also of anxiolytics, hypnotics, and sedatives in the National Health Service (NHS), projecting a 50% reduction in the increasing prescription pattern, which was not verified 20. The 2020 review of this document set as a goal to reverse the trend in the prescription of BZDs in the population by stabilising them 21, but recent data show a consistent increase in consumption 22. It is also a documented fact that BZDs are mostly prescribed in primary health care settings, both in Portugal and internationally 23,24, pointing to the crucial necessity to implement interventions aimed at changing BZD prescription patterns in such settings.
A large number of interventions using different methodologies have been implemented to change BZD prescription patterns in primary health care settings, such as minimal educational interventions 25-27, systematic discontinuation interventions 28,29, audit and feedback interventions 30, and policy interventions 31,32. The results are inconsistent; when positive, the effect is frequently lost after a short period and seems closely related to each country's primary health care setting’s particular characteristics.
An educational outreach intervention previously implemented in Portugal had a small effect on BZD prescribing trends and pointed out as a primary limitation the lack of staff to implement face-to-face educational sessions to a larger number of general practitioners (GPs) with adequate frequency 33. Online interventions, namely targeting behavioural change, with a low cost and high possibility of wide distribution, were considered a possible way to surpass limited time and resources in primary health care settings to implement an intervention of this nature.
BZD prescription has been reported as a complex behaviour influenced by GPs’ values, beliefs, attitudes, and experiences, as well as patients’ characteristics and demands 34-36. Therefore, besides the relevance of adequately implementing an intervention aimed at changing BZD prescription patterns, it is also crucial to gather information about the barriers to this implementation and consider influencing factors such as acceptability, practicability, effectiveness, affordability, and equity. This study aimed to evaluate the impact of a Digital Behaviour Change Intervention (DBCI) in the form of a tailored online program called ePrimaPrescribe, in terms of changing BZD prescription patterns and, at the same time, to test its implementation in a real-world situation.
Objectives
Main Objective
To evaluate the effectiveness of a DBCI in a primary health care setting, in which GPs were given access to an online program, ePrimaPrescribe, aimed at reducing BZD prescription.
Secondary Objective
To analyse the effect of ePrimaPrescribe on antidepressant prescriptions. Also, to study the effect of ePrimaPrescribe on the monthly registration distribution of psychological symptoms, complaints, and diagnoses coded in the same month as BZD and antidepressant prescriptions. The aim was to verify if after the intervention implementation there was a more accurate choice of diagnosis coding associated with each prescription type. Thirdly, to perform a cost analysis considering the monthly NHS spending on BZD co-payments. Finally, to analyse the implementation process using quantitative and qualitative methods.
Materials and Methods
Study Design and Setting
We chose an effectiveness-implementation hybrid design taking a dual focus a priori on assessing clinical effectiveness and implementation 37. A hybrid type 1 study tests a clinical intervention, while gathering information on its delivery during the effectiveness trial, and its potential for implementation in a real-world situation. As the methodology for the intervention of our hybrid type 1 effectiveness-implementation trial, we chose a two-arm cluster-randomised clinical trial. The cluster design was selected because both the allocation and intervention were implemented at the health care unit level.
The setting for our intervention was primary health care units in the rural Central Alentejo region of Portugal, with an area of 7,393 km2, with an estimated population of 166,706 inhabitants (2011), a population density of 22.5 inhabitants/km2, and approximately 250 GPs working in primary health care units. In Portugal, the NHS distinguishes two model types of primary care units. The default one is the “personalised care units” model (UCSP), in which professionals receive a fixed salary. The other model is the “family health units” model (USF), which enjoys higher functional and organisational autonomy 38, and where GPs might have a mixed payment scheme that includes salary, capitation, and pay for performance 39. Portugal has a public and accessible NHS, but mental health indicators are nevertheless alarming 16. BZD prescription in Portugal is very high, as introduced in our background section, and the consumption of these drugs is particularly significant in the region where our intervention was implemented 17.
Eligibility Criteria and Recruitment
All primary health care units from the Central Alentejo region were considered eligible. We contacted each health care unit coordinator, explained the project, and invited their participation. The primary inclusion criteria were health care units where at least 90% of GPs agreed to participate in the study. We chose a higher acceptance rate than usually reported in the literature since we expected a significant number of GPs in each cluster, even though they had agreed to participate in the study, would not actively use the DBCI ePrimaPrescribe platform.
Sample Size
The unit of observation for primary analysis was at the individual GP prescription level. Data collected from a previous research implemented in a similar setting 40 allowed us to perform an estimation for sample size (SS) calculation. We started by doing an SS calculation based on the independence of all observations and an effect size of 20% reduction. We obtained a total of n = 58 participants, with n = 29 participants per study arm. To adjust for the intra-cluster correlation coefficient (ICC) 41, we then calculated the design effect (Deff) expressed as: Deff = 1 + r(m - 1), where r denotes the ICC and m is the size for each cluster. Considering an average cluster of m = 5 and an ICC of 0.02, Deff = 1.08.
The SS adjusted for ICC was then be given by: n* = n[1 + r(m - 1)], so n* = 29[1 + 0.02(5-1)] = 31.3, hence approximately 32 per study arm. The number of clusters (k) was given by: k = n[1 + r(m - 1)]/m, so k = 6.4 ≅ 7.
Considering an ICC of 0.02, a 1:1 ratio of allocation of controls per intervention unit, an alpha type error of 0.05, a cluster size of 5 doctors per unit, a minimal difference 20% reduction in the number of BZD prescriptions, the study would have to include 7 clusters per study arm to have an 80% power.
Random Allocation
Randomisation was stratified according to the organisational type of primary health care unit (UCSP vs. USF), the number of GPs per unit, the average number of appointments, and the number of patients per unit per month. We further included in our randomisation criteria primary health care units’ proximity to one another, meaning that when distinct units functioned in the same location/building, we allocated them to the same study arm.
In previous research, using similar baseline BZD prescription data from primary health care units, there was a large spread of monthly BZD prescriptions 40. Since this spread was similar throughout different units, no stratified randomisation (or matching for similar prescribing patterns) was considered necessary 42.
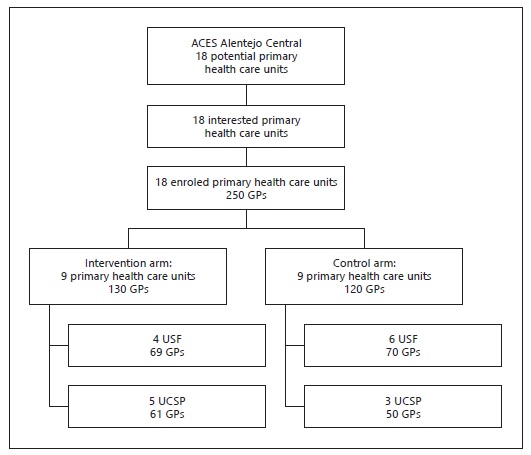
Health care units were included if 90% of the GPs agreed to participate. For concealment of allocation, after all eligible health care units have agreed to participate, we allocated them simultaneously to the intervention or control group using a computer-generated random number of tables. The distribution of participants after randomisation is presented in Figure 1.
Blinding
GPs could not be blinded since they were asked to participate actively in a study seeking to change their prescription practices. GPs included in both the intervention and control arms were asked to access an online platform. However, they were blinded to the existence of alternative content on the platform presented in their primary health care unit. The authors were not blinded to primary health care unit allocation.
The primary and secondary outcomes were assessed through analysis of the electronic prescription and diagnosis registration database for each GP. These data were extracted and anonymised (except for primary health care unit identification) by a data manager at the central Shared Services of the Portuguese Ministry of Health, who was not involved in the study and was blinded to participant allocation.
Intervention
Development of DBCI Online Programs
The DBCI online programs design was based on the Behaviour Changing Wheel theory 43, considering that behaviour change is key to improving health care and health outcomes. We used a DBCI mode of delivery. The ePrimaPrescribe program, designed to be implemented in the intervention group, was developed based on guidelines for anxiety and depression treatment and BZD withdrawal. Our primary sources of information were the National Institute for Health and Care Excellence (NICE) guidelines 11, guidelines issued by the Portuguese National Health Directorate 44, and other relevant literature specifically addressing de-prescribing and evidence-based practice guidelines 45,46. The program comprised three e-learning modules, each of approximately 30 min duration and with the following subjects: pharmacological effect and clinical use of BZDs; how to treat anxiety disorder avoiding the continuous use of BZDs; how to manage BZD dependence and BZD withdrawal proposals.
The primary health care units included in the control arm of our study were also offered an online platform, named ComunicaSaudeMental. This DBCI platform’s content was developed based on literature concerning general communication techniques and more specific communication techniques for addressing light to moderate mental health disorders or patients’ emotional management in primary health care settings 47. This program comprised (similarly to the one offered to the intervention arm) three e-learning modules, each of approximately 30 min.
After the development phase, modules were uploaded to two moodle platforms (one for the intervention educational platform, ePrimaPrescribe; another for the control educational platform, ComunicaSaudeMental), and individually coded access was generated for each participant. Before the implementation phase, both programs were tested by 3 GPs and 3 psychiatrists. Two of the GPs were members of the Primary Health Care Research Department at NOVA Medical School, and one was also a member of the Mental Health Research Department at NOVA Medical School. Two of the 3 psychiatrists were specialists in substance misuse. These experts were asked to comment critically on the accuracy and quality of the e-learning modules. Their suggestions were carefully considered, and when appropriate, were integrated into the module’s final version.
Intervention Implementation
We used the Template for Intervention Description and Replication (TIDieR) checklist and guide 48 as guidance to the following intervention implementation report. The ePrimaPrescribe online platform was implemented in primary health care settings. A DBCI was chosen as the delivery method since we hypothesised that a free, easily accessible platform might have a higher and longer-term effect in terms of changing BZD prescription patterns.
Primary health care units allocated to both the intervention and control arms had an initial face-to-face visit where we presented the online platform to the GPs, delivered their identification access codes, and explained how to access and use the DBCI. Written informed consent was confirmed during this initial face-to-face visit. We performed all face-to-face visits to the allocated primary health care units within a 6-week time frame.
We expected GPs to start accessing and hence actively participating in the study in the weeks following the first face-to-face visit to their unit. Access to the platform was available at any convenient time and frequency, through any digital device with an internet connection.
We sent an email to GPs every 3 months, at 3, 6, and 9 months after implementation, as a reminder of their participation and as a strategy to improve adherence to the intervention. The platform was not tailored, personalised, adapted, or modified in any way during the intervention implementation period.
To assess how well the intervention was implemented, and hence to evaluate the extent to which the intervention was delivered as planned, we performed another face-to-face visit to all participating primary health care units 12 months after the initial intervention. During this visit, GPs included in the intervention arm were asked to answer a survey exploring their motivations and expectations regarding the use of the ePrimaPrescribe online program. They were also asked to complete a questionnaire evaluating the barriers and facilitating factors to the implementation of ePrimaPrescribe and to participate in an exploratory group discussion on their general perceptions of their study participation and platform implementation.
All face-to-face visits, and hence the explanation of the intervention procedures and monitoring, were conducted by the first author, who has a background as a psychiatrist and regularly performs her clinical work in the same geographical area where the intervention was implemented. We performed semi-structured in-depth interviews with an intentional sample of participants from the intervention arm to explore perceptions on the feasibility and implementation of the study.
Outcome Assessment
Primary Outcome Measure
As the primary outcome measure, we used the number of BZD prescriptions issued per month, specifically, the proportion of prescriptions issued by participants included in the intervention and control units over the study time frame, and more specifically at baseline, 6, and 12 months after the intervention. We included BZDs from the following Anatomical Therapeutic Chemical classification system-coded groups: N05B, N05C, and N03AE [49].
Secondary Outcome Measures
Effectiveness. As the secondary outcome measure, we used the number of antidepressant prescriptions issued per month; specifically, the proportion of prescriptions issued by participants included in the intervention and control units over the study time frame and more specifically at baseline, 6, and 12 months after the intervention. We included antidepressants from the following Anatomical Therapeutic Chemical classification system-coded group: N06A 49. To study the effect of ePrimaPrescribe on diagnosis registration, we used the monthly registration distribution of psychological symptoms, complaints, and diagnoses coded in the same month as BZD and antidepressant prescriptions. The GP diagnosis registration used the International Classification of Primary Care, second edition (ICPC-2), developed and updated by the World Organisation of Family Doctors (WONCA) International Classification Committee (WICC) 50. We further performed a cost analysis considering the monthly NHS spending on BZD co-payments. This cost was compared with the cost for implementing psychological interventions to treat anxiety and depressive disorders in primary health care settings.
Implementation
We studied the implementation process using quantitative and qualitative methods.
Quantitative Data. We developed a standardised onsite survey aimed at exploring the following themes: GPs’ self-evaluation of their knowledge about the management of patients with anxious and depressive symptomatology; their reasons for prescribing BZDs and antidepressants; their subjective assessment of the amount of BZDs and antidepressants prescribed; their reasons for continuing long-term BZD prescription; their difficulties in changing long-term BZD prescription; their degree of concern with continued BZD prescription; their knowledge of and degree of adequacy regarding the existing Portuguese guidelines on BZD prescription; their motivations and expectations regarding the use of the ePrimaPrescribe program in clinical practice; and their participation in the study. This questionnaire had 18 multiple choice questions and 14 short answer questions. We adapted the Barriers and Facilitators Assessment Instrument (BaFAI) 51 to the implementation of the DBCI ePrimaPrescribe program. This questionnaire was organised into four categories: barriers deriving from the characteristics of the practice/innovation; barriers deriving from the characteristics of the professionals; barriers due to patient characteristics; and barriers arising from the intervention context. The questionnaire had 25 questions using a five-point Likert scale. We asked all participating GPs to complete the onsite survey and BaFAI questionnaires at the end-of-study face-to-face visit that was performed after completion of the 12-month intervention period. During this visit, we also collected GPs’ sociodemographic data.
Qualitative Data. During our end-of-study face-to-face visit, we facilitated a group discussion in each primary health care unit included in the intervention group, to explore GPs’ perceptions of their participation. Finally, we performed semi-structured in-depth interviews with an intentional sample of participants from the intervention arm to explore perceptions of the study’s feasibility and implementation. The interview guide structure was developed after an exploratory analysis of the themes emerging from the answers to the onsite survey questionnaire and the BaFAI, and from the group discussion during the end-of-study face-to-face visit.
Data Management
Each prescription data was coded using an individual GP and patient numerical identification, in a secured and validated electronic database, directly extracted by the central Shared Services of the Portuguese Ministry of Health. Data concerning clinical diagnosis was extracted using the same method. Matching of the prescription and diagnosis databases was performed using the coded patients’ numerical identification and prescription/diagnosis registration month. Data obtained through questionnaires and interviews were collected after participants gave signed informed consent during the initial implementation face-to-face visit.
Data Analysis
We performed an exploratory descriptive analysis using the number of prescriptions as the primary outcome measure, considering the patients’ age and sex to be the main influencing factors, by units of intervention and control. We performed the most analysis at the level of intervention versus control clusters (so we compared the set of intervention units vs. the set of control units), since the available data did not allow for the author to identify each of the participating GPs, and hence also did not allow us to distinguish within the intervention units which GPs were compliant with the intervention (i.e., the GPs that used the DBCI), and which, although they initially agreed to participate, ultimately did not use the platform.
We tested for significant differences among the baseline characteristics of the intervention and control groups. We performed descriptive analysis, with continuous variables summarised using means and standard deviations for normal distributions, and medians and the 25th and 75th percentiles for non-normal distributions.
Estimated effects were calculated by comparing the number of prescriptions in the intervention and control groups at baseline, 6 months, and 12 months after the intervention. All analyses were performed on an “intention-to-treat” basis (i.e., all initially enrolled GPs were included in the analysis according to the group to which they were assigned). This approach reduced the bias that may occur when participants not receiving assigned treatments are excluded from the analysis.
We tested for significant differences in the baseline characteristics of the control and intervention groups using t-tests or one-way ANOVA. This included calculation of means and/or proportions with confidence intervals, and robust standard deviations (to account for clustering).
We performed a secondary analysis where we explored the association between the frequency of BZD and antidepressant prescription with diagnoses using χ2 tests, to test independence in two-way contingency tables. The Cochran-Armitage trend test was employed to assess how the proportion of two ordinal successes varies across the levels of a binary variable. When both variables in a contingency table had ordered categories, the linear-by-linear test was used instead 52.
We finally performed a cost analysis considering the monthly NHS spending on BZD co-payments, using t-tests or one-way ANOVA. Statistical significance was considered for p values <0.05.
R statistical software 53,54 was used to perform all the statistical analyses within the RStudio integrated development environment for R (RStudio Team, 2019). The graphs and plots were obtained using the ggplot2 R package 55.
We performed a descriptive analysis to correlate data from the onsite survey questionnaire and BaFAI questionnaire with GPs’ sociodemographic characteristics using χ2 tests for testing independence in two-way contingency tables.
We used qualitative methods, namely content analysis, to explore data from the in-depth interviews using ATLAS.ti 56. Interviews were coded, synthesised, and categorised according to similarities of meaning 57,58. Patterns within and across categories were analysed and grouped into themes. Categories and themes were driven by literature concerning the problem of excessive BZDs prescription, on interventions to change prescription patterns, and barriers and facilitators to such changes. Coding continued until no new concepts emerged from the data. Coding, category-building procedures, and thematic analysis were discussed by the authors until consensus was reached. Data were reported elsewhere according to the 2010 Consolidated Standards of Reporting Trials guidelines.
Discussion
Strengths and Limitations
The concerning reality of excessive BZD prescription in Portugal 14-16 suggests the need for effective interventions, at minimal cost and with a low need for professional time. A maximally effective withdrawal strategy is especially important in primary care settings because of budgetary limitations and the small amount of GP time available per consultation 59.
BZD prescription is considered a complex behaviour 34-36. Behaviour change is key to improving health care and health outcomes 60. Therefore, we chose to design our intervention based on a behaviour change theory, the Behaviour Changing Wheel theory 43, in the hope that using a validated and reviewed framework would strengthen the quality of our approach.
Concerning our effectiveness trial, this paper describes the protocol for a cluster-randomised trial to assess, primarily, whether a DBCI would have a significant effect on BZD prescription. It also describes two secondary analyses regarding antidepressant prescription trends and reporting of diagnosis registrations associated with BZD prescription, in the hope that the implementation of our DBCI could also translate into improved anxiety disorder registration and adequate treatment (with an increase in antidepressant prescription).
Randomised trials are the gold standard to assess intervention effects, and cluster-randomised trials are an appropriate design when interventions need to be introduced to groups of individuals (in our case, groups of GPs prescribing in the same primary health care unit), which are randomly allocated to different study arms 41,61.
We chose a DBCI as the delivery method because these online interventions have the potential for low unit costs, high reach, and maybe an effective and acceptable way to benefit individuals and society 62. We chose to include both prescription-related outcomes and diagnosis outcomes. We recognise the limitation of presenting our prescription-related outcomes as the frequency of prescription. We intended to use the World Health Organisation-recommended outcome measure, Defined Daily Dosage (DDD) per 1,000 inhabitants per day (DHD), which provides a measure of exposure or therapeutic intensity in a defined population, allowing comparisons across various periods and population groups; however, the database provided by the Shared Services of the Portuguese Ministry of Health did not allow for such analysis. Notwithstanding, in the specific case of BZDs in Portugal, we consider this limitation was minimised by the fact that BZD prescriptions cannot be placed in chronic repeated prescriptions. This means that when a BZD prescription is issued, it has to be dispensed in the next 30 days and that each prescription can have a maximum of two packages of the drug. Hence, since there is the need for frequent prescription renewal, we considered that the number of prescriptions issued was an adequate measure to estimate our intervention effect.
Regarding clinical diagnosis outcomes, we recognise that our data might reflect our fairly inexact approach since most prescriptions are not associated with psychological symptoms, complaints, or diagnoses registration, and also because even when this association was found, it did not mean that symptoms or disorders were identified at the moment of prescription. In Portugal, it is not mandatory to perform a complaint/symptom/diagnosis coding each time a GP issues a prescription. The registration tool available in the Portuguese primary health care units allows GPs to code just once a certain diagnosis and keep it associated with the patient file, hence dismissing further need to repeat the diagnosis coding, no matter how long the treatment for that disorder is kept. Despite this limitation, we considered it relevant to report our analysis of changes in clinical diagnosis, since it might indicate significant secondary effects coming from the intervention implementation.
We recruited GPs to participate, considering all the available characteristics that might influence our primary and secondary outcomes: sex, years of clinical experience, type of primary health care unit, and previous training in mental health.
We recognise as a limitation and possible bias the fact that it was the first author who was mainly responsible for implementation and at the same time responsible for collecting data from the questionnaires and in-depth interviews. We minimised this limitation by having all data, including its categorisation, content, and theme identification, reviewed by a researcher who was not involved in data collection.
Relationship to Other Studies and Expected Contribution
Excessive BZD prescription is a reality for many countries other than Portugal. For this reason, a large body of evidence already exists, with extended research assessing the effectiveness of interventions aimed at changing BZD prescription patterns. From simple methodologies, such as sending letters to long-term BZD users, to more complex ones, such as multi-step tailored interventions, most studies show limited short-term effects. Most studies also lack a more profound understanding of the facilitators and barriers to implementation.
We consider our trial to be innovative since it presents a methodology that was carefully prepared to reach maximum effectiveness, at minimal cost and with a low need for professional time, and it also has an important focus on factors influencing implementation. We also consider our research to be of particular national interest because no matter how well structured an intervention might be if it is not accepted by the public for whom it is designed then the results will inevitably be limited.
In dedicating a significant effort to explore GPs’ perceptions of their participation experience, we expect to contribute to two important areas of knowledge. On theone hand, to a better understanding of the factors influencing the act of BZD prescription, and possibly in a larger perspective, what this prescription means/represents in the management of mental health issues in primary health care settings. On the other hand, in a time and setting where online interventions are becoming more common, we aim to explore the factors influencing the acceptance and practicability of our DBCI. Concerning this mode of delivery specifically, we also expect our research to contribute to an in-depth exploration of perceptions that are liable to be applicable in other areas besides mental health, and hence it could help to shape digital interventions according to what GPs want - and thus they will feel more motivated to comply.
Acknowledgements
We would like to thank Ricardo Vicente e Cátia Pinto from SPSM services for facilitating access to data extraction, all of the coordinators of the primary health care units in the Central Alentejo region for facilitating the study implementation, and all the GPs who willingly participated in the study and openly shared their perspectives.
Statement of Ethics
This trial was approved by the Ethics Commission for Health of the Regional Administration of Health for Alentejo Region [Portugal; 02/2016(CES)] and the Nova Medical School, Nova University Lisbon, Portugal Ethics Commission (47/2016/CEFCM). The results of this study were disseminated via peer-reviewed publications and conference presentations.