Introduction
Widely used since ancient times for its important chemical and physical characteristics 1,2, asbestos is recognized in all its varieties as a human carcinogen by the International Agency for Research on Cancer (IARC) 3 and as the leading cause of cancer-associated occupational exposure in industrialized countries 3-5. Since 2004, the World Health Organization (WHO) estimates that approximately 125 million individuals were exposed to asbestos at their workplace and that 107,000 deaths and 1,523,000 disability-adjusted life years occurred each year as a result of diseases related to their exposure 6,7. Asbestos can cause diseases such as lung, laryngeal, ovarian, and gastrointestinal cancer, asbestosis, and mesothelioma 8-10, but it is malignant pleural mesothelioma (MPM), of monofactorial origin, that has been assumed to be the determining factor in the study of this subject 5,8,11,12. Although the relationship between asbestos exposure and MPM is well known 13, the most studied occupational agent since 1965 with more than 12,000 bibliographic references on MEDLINE, many of the published studies is related to litigation against asbestos manufacturers, suppliers, and providers of asbestos-containing products 14 leading to the fact that much is still unknown about asbestos consumption and its location, as well as about incidence and mortality from MPM.
Increased incidence and mortality rates from MPM reflect the massive use of asbestos in industrialized countries in the past but also the current production and consumption in many developing countries 13,15. Despite all the efforts to ban its use, to date, only 67 countries have banned its use 16. In the USA, it remains legal with an estimated consumption, between 2016 and 2020, of 535 tonnes 17. In 2016, India and China were the world’s leading asbestos consumers (308.000/288.000 tonnes, respectively) and, in 2018, Russia represented the largest producer of asbestos used worldwide (710.000 tonnes) 16. For this reason, it is also estimated that diseases related to this exposure, notably MPM, will continue to be a major health problem for many decades to come, increasing 5-10% per year, in industrialized countries, in a heterogeneous manner 8,15,18.
In order to define directives to raise awareness and to allow a public institution to plan and aim for its elimination, as a response to the joint work of the International Labour Organization (ILO) with the World Health Organization (WHO), which led to the Parma Declaration in 2010 19, many countries have already set up ongoing epidemiological surveillance projects of both mortality and incidence of this disease 20 (National Mesothelioma registries in Australia, France, South Korea, and Italy 21 and mesothelioma mortality in UK 22, US 23, Spain 24, Greece 25, Brazil 26, and Italy 27). This systematic review aims to map the relationships between asbestos and MPM by studying the exposure to asbestos and the incidence and mortality of MPM. By mapping these aspects, we can contribute to bringing to the fore the actuality of this problem and consequently to the need to broaden the focus of research and implementation of National Mesothelioma Surveillance Centres at a global level.
Methods
This systematic review was reported following the PRISMA 2020 guideline for reporting systematic reviews 28.
Protocol and Registration
A protocol was developed using the guidelines of the Joanna Briggs Institute (JBI) approach to evidence-based healthcare - systematic reviews of aetiology and risk 29 - and it was registered in the Prospero database (CRD:42021242963, https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021242963), prior to conducting the search.
Eligibility Criteria
This review includes all studies that address the relationship between asbestos and MPM in terms of exposure to asbestos, incidence, and mortality by MPM. Studies presenting data on other types of mesotheliomas were also accepted, provided it was possible to exclude data relating to MPM. We restricted our search to studies written in English, French, and Portuguese.
All studies that focus on humans diagnosed with MPM by exposure to asbestos, regardless of age and gender, were included. All studies in occupational and non-occupational contexts were also included. The relationship between exposure to asbestos and MPM was analysed in an occupational and non-occupational context because, although occupational exposure is the most frequent 13,15, this relationship has also been demonstrated in non-occupational exposure, namely, in women 30,31. Only studies in which exposure was confirmed and that only pertained to asbestos, regardless of dose and exposure time, were included.
Search Strategy
Systematic searches were conducted from September 11 to 15, 2021. We searched for eligible published and unpublished studies in databases and sources of grey literature. The databases included were PubMed (using the terms MESH), the Web of Science, and the Cochrane Library, as well as the sources of grey literature, i.e., the Open Access Scientific Repositories of Portugal, DART-Europe E-theses Portal, from January 1, 1960, to December 31, 2020. We extended our research to 1960 since that was the year in which the first study demonstrating a causal relationship between asbestos exposure and MPM was published 1,2,14,32 and also marked the period of increased incidence of and mortality from malignant mesothelioma 33.
Study Selection
Databases searches were saved, and duplicated records were identified and removed. Two independent reviewers (CS, MAD), from a group of five (CS, MAD, ESL, PA, and ASU), screened titles and abstracts. A third reviewer helps to resolve disagreements (ASU). After potentially eligible studies had been selected, two independent researchers reviewed the retrieved full-text studies for eligibility; one researcher (CS) screened all studies; and four researchers (MAD, ESL, PA, and ASU) collectively screened the same studies for agreement; a third reviewer (ASU) read studies to resolve disagreements. The reference lists of identified studies were reviewed for additional relevant studies. Several study authors were contacted to assess to the full-text article.
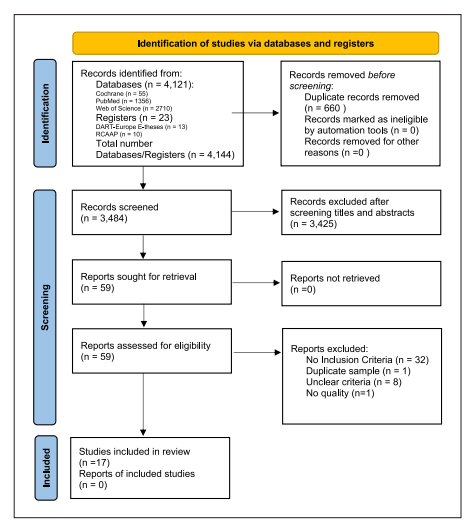
After the studies were screened, there was further application of the critical appraisal checklist for cohort studies (online suppl. Appendix 1; see https://www.karger.com/doi/10.1159/000527971 for all online suppl. material), case-control studies (online suppl. Appendix 2), and analytical cross-sectional studies (online suppl. Appendix 3) 29. A PRISMA flow diagram of included studies was performed. A list of excluded studies and reasons for exclusion was also performed and generally presented in the PRISMA flow diagram (Fig. 1).
Data Collection Process
Both reviewers independently extracted study data into a prepared spreadsheet (Excel®, Microsoft Corporation, Redmond, WA). The data consisted of the last name of the first author, publication date, country of origin, language, primary aim, method, sample size, gender, age, period/time of study, country in study, data source, exposition characteristics (type, history, exposure time, age at first exposure, via, latency, fibre type, fibre dimensions, cumulative exposure), incidence (age at incidence, diagnosis year), and mortality (number of deaths).
Two reviewers independently extracted data from each article using the constructed form. To ensure consistent data extraction by all reviewers, we pilot the form in five studies. During the pilot, reviewers clarified differences in interpretation and the standardized data extraction. After the pilot, studies used are randomly assigned and screened again during the data extraction. One researcher (CS) extracted data from all studies, and five researchers (MAD, ESL, PS, and ASU) collectively extracted data from the same studies. Any disagreements in data extraction were settled by consensus among each pair of reviewers. The information entered on the form was subsequently analysed.
Risk of Bias (Quality) Assessment
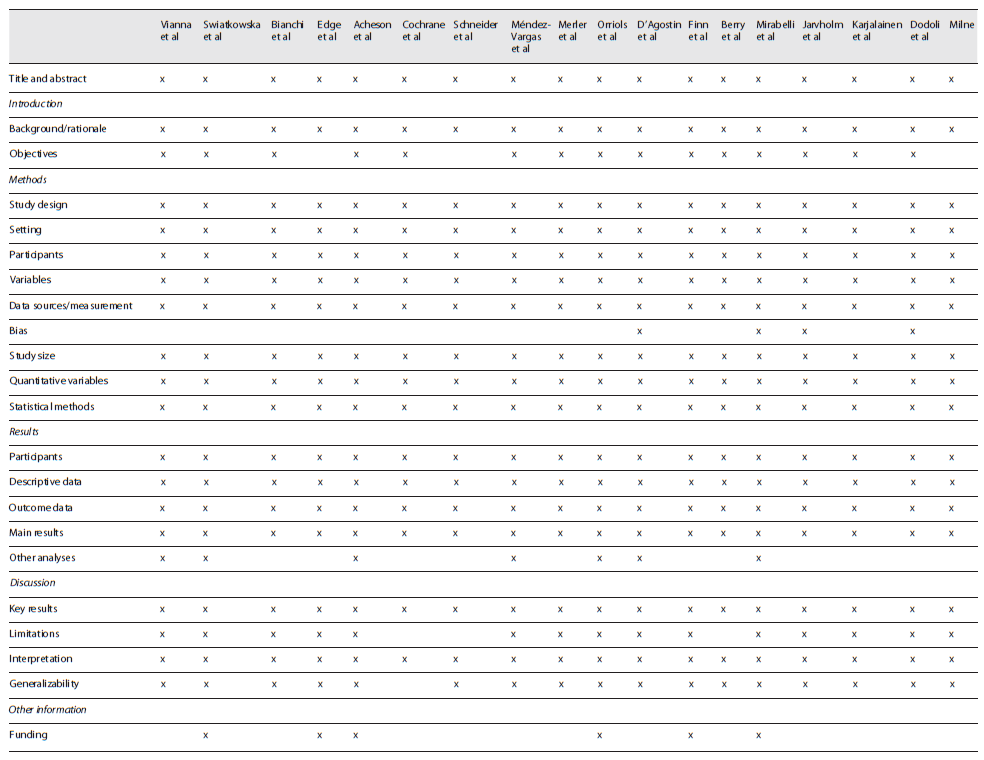
To minimize the risk of bias and increase the quality of this review, only studies related to MPM, the only disease known to date to be monofactorial after exposure to asbestos 5,8,11,12, were included. Data were always reviewed by two reviewers, and a third reviewer read studies to resolve disagreements. To assess the quality and strengthening of the studies 34, the STROBE checklist was applied (STROBE checklist: cohort, case-control, and cross-sectional studies).
Results
Study Selection
The databases search yielded 4,121 hits (PubMed [n = 1,356], Web of Science [n = 2,710], and the Cochrane Library [n = 55]) and the registers search yielded 23 hits (Open Access Scientific Repositories of Portugal [n = 10] and DART-Europe E-theses Portal [n = 13]) (Fig. 1). Assessment of selected studies’ reference lists identified no additional eligible studies. A total of 3,484 unique papers were identified and screened by title and abstract; 3,425 papers were excluded for not presenting inclusion criteria. Fifty-nine papers were read to determine eligibility for inclusion. Seventeen met the inclusion criteria and were included in the review (Table 1), while 42 papers were excluded (32 for not presenting inclusion criteria, 8 for unclear criteria, 1 for duplicate sample, and 1 for lack of quality) (Fig. 1).
Study Characteristics
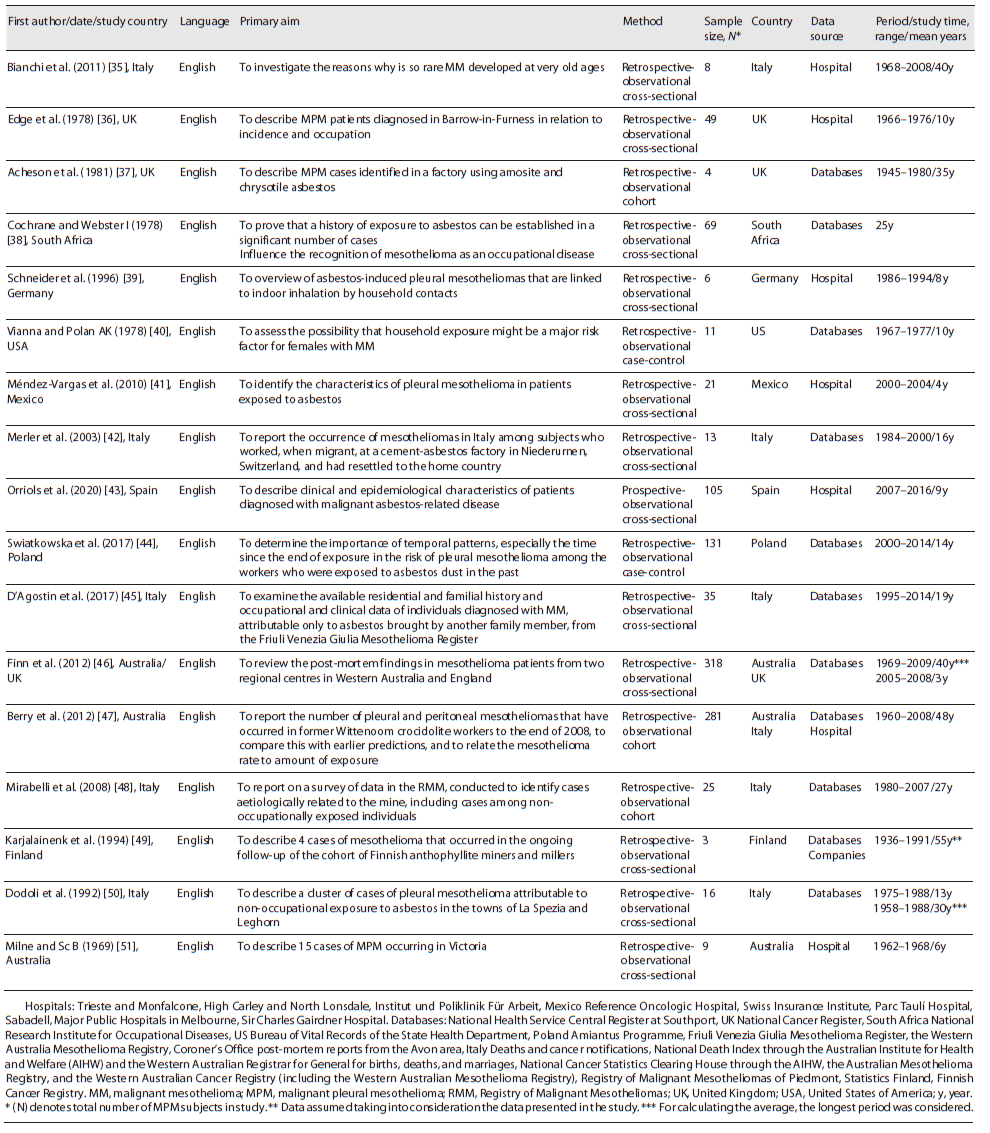
Of the seventeen studies identified, sixteen were retrospective and one was prospective. They were all observational studies (twelve analytical cross-sectional, three retrospective cohort, and two retrospective case-control). All were published in English, between 1969 and 2020, Italy being the country of origin of most studies. The identified studies included 1,104 participants. Eleven of the studies were conducted in Europe, one in South Africa, one in the USA, one in Mexico, one in Australia, and two were conducted jointly between Australia/UK and Australia/Italy. Studies were conducted in occupational and non-occupational contexts. In five studies, the data presented refer to hospital data only, ten studies to data obtained from national databases only, one to data obtained from national databases and mining company, and one to data obtained in national databases and hospital. The overall studied period was on average 24 years for the retrospective studies and 9 years for the prospective study, with a global range between 1936 and 2016.
Methodological Quality/Risk of Bias
The result of the appraisal of each study is presented in Table 2. The specific STROBE checklist used includes a number of questions that pertains to combined case-control, cohort, and cross-sectional studies 34. The studies were all observational. The fact that the research was extended to 1960 means that many studies were not designed according to current quality and strengthening guidelines. Therefore, in general, studies had limited enrolment, two of them did not present the objectives described in the introduction, three did not present study limitations, four explicitly specified the risk of bias, and only six referred to fundings.
Findings of Individual Studies
Table 1 shows the summarized findings of the included studies’ characteristics. Patients’ characteristics, asbestos exposure, and incidence and mortality to MPM are described in more detail below and summarized in Table 3.
Characteristics of Participants
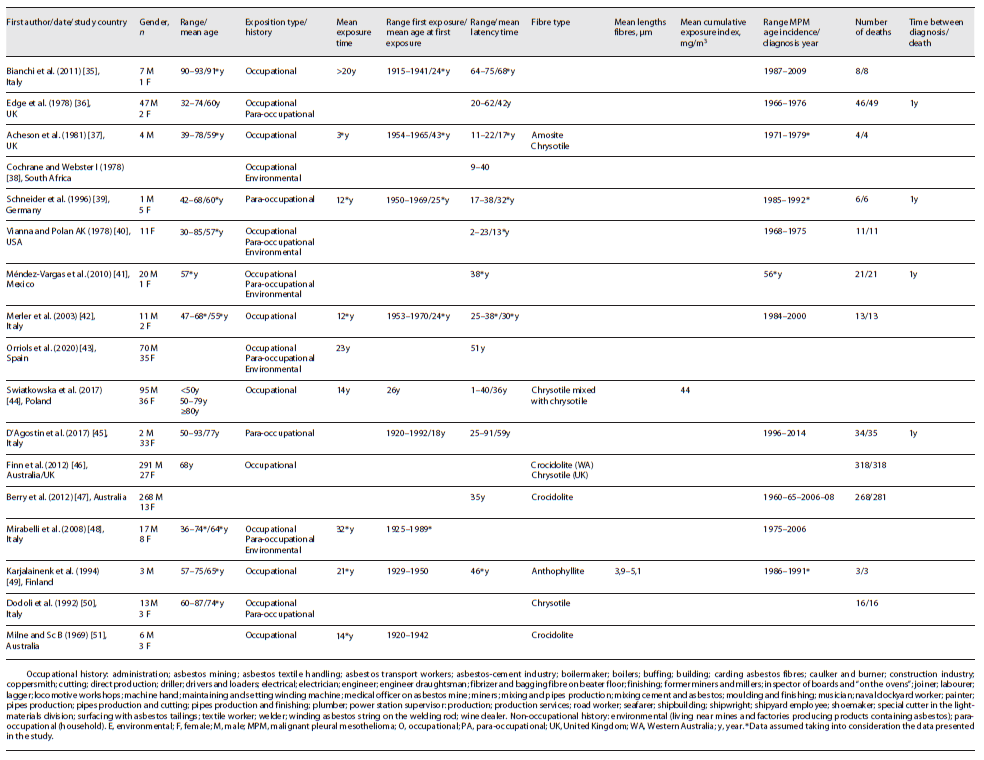
Of the 1,104 patients under study, 855 were male, 180 were female, and 69 were unknown. In only twelve studies (five occupational, five combined occupational and non-occupational, and two para-occupational) was possible to calculate the average age, which was approximately 66 years, with a range between 30 and 93 years. Four studies (one occupational, two combined occupational and non-occupational, and one unknown) did not present data regarding the age of patients and one study (occupational) presented data regarding classes <50 years, 50-79, and 80 years. All cases of MPM included in the study were validated clinically and/or histologically.
Characteristics of Exposure
Seven studies were related to occupational exposure, two to non-occupational exposure (para-occupational), seven to combined occupational and non-occupational (environmental and para-occupational) exposure, and one was unknown. In all studies, inhalation was via exposure. The average exposure time was 16 years in eight studies (five occupational, two combined occupational and non-occupational, and one para-occupational), with a range of 3-32 years. One study (occupational) presented a mean time of exposure >20 years and in eight studies (one occupational, five combined occupational and non-occupational, one para-occupational, and one unknown), it was unknown.
In eight studies (five occupational, one combined occupational and non-occupational, and two para-occupational), it was possible to determine the interval period of the first exposure, 1915-1992. The mean age at first exposure was approximately 27 years (obtained in six studies - four occupational and two para-occupational). The mean age at first exposure was 29 years in occupational studies and 22 years in para-occupational studies. The mean age at first exposure could not be obtained in combined occupational and non-occupational studies. The exposure happened between 1915 and 1970 in occupational exposure, 1920-1992 in para-occupational exposure, and 1925-1989 in occupational and non-occupational exposure. The exposure time was higher in the studies carried out in combined occupational and non-occupational studies, namely, 28 years, whereas it was 13 years in occupational studies and 12 in para-occupational.
Only in seven studies (five occupational, one combined occupational and non-occupational, and one unknown context), the type of asbestos fibre to which the patients were exposed is presented, namely, chrysotile, crocidolite, amosite, and anthophyllite. Chrysotile was identified in four studies and crocidolite in three studies. Two studies were conducted in the UK, where chrysotile was the type of asbestos under study, and three in Australia, where the type of asbestos was crocidolite. Only one occupational study had data on mean cumulative exposure index - 44 mg/m3 - and another occupational study had mean lengths fibres of 3.9-5.1 μm. Of the nine non-occupational studies with data concerning the non-occupational context, eight referred to para-occupational exposure (household) and five to environmental exposure (living near mines and factories producing products containing asbestos). In fourteen studies concerning occupational exposure, shipbuilding, construction, production of asbestos-containing products, and mining were the main occupations of the patients.
Mesothelioma Latency Period
In twelve studies (five occupational, four combined occupational and non-occupational, two para-occupational, and one unknown), the average latency period was approximately 42 years, with a minimum latency time of 1 year and a maximum of 91 years. The latency period is 46 years in the para-occupational studies (between 17 and 91 years), 39 years in occupational (between 1 and 75 years), and 36 years in the studies carried out in combined occupational and non-occupational (between 2 and 62 years).
Ten studies (four occupational, three combined occupational and non-occupational, two para-occupational, and one unknown) presented the period of incidence and, in general, the first case was identified in 1966 and the last in 2014. For occupational context, the range of diagnosis was 1984-2000, in combined occupational and non-occupational it was 1966-2006, and in para-occupational it was 1985-2014.
Mesothelioma Incidence by Sex and Age
From the data that can be extracted from occupational studies and non-occupational studies, it is possible to see that, in occupational studies, men had a higher incidence of MPM, varying between 67 and 100% of the total number of patients included. Nevertheless, in para-occupational studies, women had a higher incidence (>83%). In the combined occupational and non-occupational studies, the incidence rate was also higher in men, with rates between 67 and 100%. In what regards the mean age of the patients of MPM, it can be determined that in occupational studies it was 68 years old, in para-occupational studies 69 years old, and in combined occupational and non-occupational studies it was 62 years old.
Mesothelioma Mortality
In twelve studies, it was possible to collect data on the number of patients who died by the time of the publication (five occupational, four combined occupational and non-occupational, two para-occupational, and one unknown). The mortality rate was >89%, being in nine of them, 100% (five occupational, three combined occupational and non-occupational, one para-occupational).
Only four studies (two occupational and non-occupational and two para-occupational) presented data regarding the interval between the time of diagnosis and death, which is approximately 1 year. In five studies, the data were obtained post-mortem, and in eight it was unknown. In occupational studies, the mortality rate was 100%, while in para-occupational studies it was 97-100%, and in occupational and non-occupational studies 94-100%.
Discussion
Summary of Evidence
In this systematic review, abundant studies were identified, reinforcing the fact that asbestos is the most widely studied occupational agent 14. All seventeen included studies are observational and heterogeneous among themselves. This can be explained by the fact that to study the association between an exposure risk and a particular disease, epidemiological studies are normally the most widely used 14. However, when it comes to the relationship between exposure to asbestos and MPM, in which much is still unknown about dose response, cumulative exposure, and exposure history, aggravated by the fact that MPM is a rare disease, difficult to diagnose, and with a long latency period, studies tend to be fraught with error and flawed in terms of bias, making these studies invalid for meta-analysis 14. Another important factor is that the studied type of exposure must be similar, which was not the case in the studies in general because of mixed contexts such as occupational and non-occupational and, within occupational, with different occupations 14: this is exactly what we found in the seventeen studies included in our systematic review, which, as may be seen in Table 3, present mixed exposure contexts.
Nevertheless, it is known that Europe is the current centre of the asbestos-related diseases burden 52, in particular Italy, which is known to be among the largest producers and users of asbestos in the 20th century, until its ban in 1992 20,53, and the highest incidence reported of mesothelioma in the world, in the Italian Province of Genoa (5.8/100 000) 54, even if the highest malignant mesothelioma incidence in the world occurs in the UK and Australia 55. These data agree with the data found in this systematic review, in which eleven of the seventeen studies were conducted in Europe, six in Italy, and two in UK. Another three were conducted in Australia 1, Italy and Australia 1, and the UK and Australia 1.
It can be stated that all individuals have been exposed to low doses of asbestos at least once in their lives 14,56. A number of studies included in this systematic review support that occupational exposure is the most frequent 57, namely, in men 58, while non-occupational exposure is more frequent among women 13, occurring in domestic settings through cross-contamination with relatives working with asbestos or dwellings containing degraded materials, polluted air from local businesses producing/handling asbestos, the handling of friable materials, contact with places where these minerals form naturally, and in natural disasters 59,60. Studies show that there is a risk of mesothelioma from all types of exposure, namely, environmental 5, the type of exposure that remains a major issue 61 because of the difficulty in quantifying it. The data collected in the five studies included reinforce this situation, insofar as exposure related to living near mines and factories manufacturing products containing asbestos means that this exposure is often deemed indirect and subjective, even by the individuals themselves, who usually state that they have never been exposed to asbestos.
Exposure, previously linked, in the post-World War II context, to asbestos mines and the manufacture of products containing asbestos, suffered with the cessation of the production of these products and the closure of the mines in industrialized countries such as the UK, a clear change. In this sense, it has been verified a decrease in risk for those associated with mines and manufacturing and an increased risk for those associated with construction, such as carpenters, plumbers, and other tradespeople 62, and more recently in workers who demolish, repair, or refurbish structures, plants, ships, or products containing asbestos 14. In the non-occupational context, concern has also been growing as asbestos has been identified in numerous public buildings and schools 14. Due to the long latency period and to the fact that the directives to ban and manage the use of asbestos are recent, studies included in this systematic review refer to exposures that occurred between 1915 and 1992, which does not allow to reflect and support these data.
All asbestos fibres are carcinogenic and genotoxic 4 and are not possible to define a threshold dose for exposure below which it cannot be stated with certainty that carcinogenic effects are not observed (“threshold”) 63,64. Chrysotile, crocidolite, and amosite 65 are the varieties of asbestos with the greatest carcinogenic potential for the pleura 66. Chrysotile (white asbestos), an easy-to-mould, heat-resistant, and non-acid-resistant asbestos, is known to be less toxic and accounts for 90% of asbestos used in the construction, textiles, and ceramics industry. Crocidolite (blue asbestos), resistant to acids, is the most toxic and hazardous variety, and, together with amosite, it has been the most widely used variety in the paper, board, and fibre cement industry 9,60,65,67. Only seven of the seventeen studies included presented the type of asbestos used, of which four were chrysotile, which is supported by the literature that states that chrysotile is the most common form used 68.
Inhalation is the most frequent and most damaging route to health 3,9,14,67, which is supported by the seventeen studies included in this systematic review. In the included studies, according to the literature, the latency period has been established by taking into account the time that has elapsed from the first exposure to diagnosis 69, and mesothelioma occurs even at lower doses of asbestos exposure 4,70.
The probability of developing MPM after exposure to asbestos depends on two factors: time since first exposure to asbestos and cumulative dose (fibres/mL of air x number of years of exposure) 71. No formal studies were performed of the relationship between cumulative exposure and MPM after non-occupational exposures or investigated the risk associated with asbestos materials in place in living areas 72. These data are supported by this systematic review since in seventeen studies, only one presents values related to cumulative exposure and in occupational context.
As regards the latency period, this systematic review found that the average latency period was approximately 42 years, with a minimum latency time of 1 year and a maximum of 91 years, with the first case identified in 1966 and the last in 2014. This is supported by the wider literature which demonstrates that although asbestos was banned in the European Union in 2005 19, due to the long latency period (typically 20-50 years) 4,7,8,14,66,71 between asbestos exposure and diagnosis of MPM, the incidence of MPM will continue to increase in Western Europe in the coming years 61, but it may be underreported in many countries 54.
Although in this systematic review the mean age at first exposure was approximately 27 years, because of the long latency period of MPM, another reason for its incidence to be underestimated is that, since many individuals who were older at the time of exposure may not have had time to develop the disease 14, others may die prematurely from asbestosis or lung cancer or even from other diseases, such as cardiovascular diseases 14. MPM has a very poor prognosis with a median survival from the diagnosis to death of approximately 9-12 months 73, which is supported for a number of studies included in this systematic review.
In regard to mortality from malignant pleural tumours, it was predicted that peak mortality would be reached in the early 2000 13 as well as the incidence, considering the poor prognosis of this disease 12. However, with the still current production and consumption 16,17 and the unreported cases occurring in developing countries 55, problem that lobbyists against the ban aim to keep 74, the global mesothelioma burden is unclear 75. Deaths from mesothelioma are not clearly identified in the International Classification of Diseases (ICD) until the eighth revision, which took effect in 1968 76. A specific code for MPM has been available only since the tenth revision (ICD10), which was implemented since 1994, but continues not implemented in many countries 54, which supports the statement that mesothelioma deaths being reported worldwide do not reflect the historical asbestos usage in the world 77. This systematic review reinforces the literature, as no study with the ICD10 code was identified, and only studies in which MPM was clinically and histologically validated could be included.
Another factor that might explain the fact that deaths from mesotheliomas are not clearly identified is that many studies related to mortality in MPM are based on post-mortem data, but the number of people who undergo an autopsy is not representative of the total number of people who die 14. Due to the relatively scant information provided by the included studies, it is not possible to support these data since, although we know that in four studies they were identified in life, and in five post-mortem, in eight these data are unknown. Another aspect to be taken into consideration is the fact that industrializing countries, following the path of the industrialized countries, continue to produce and consume asbestos on a massive scale, countries that are major exporters of various products to industrialized countries, such as Mexico and China to the USA 78.
Surveillance programmes are recognized for providing an understanding of the health effects of exposure to asbestos 79 but also to improve clinical outcomes such as duration of survival and quality of life and to support research to advances in the detection, treatment, and prevention 80. In that sense, a few countries, such as Italy and France in Europe, are among those that are most sensitive to the prevention and control of asbestos-related diseases, having a specific system of epidemiological surveillance of mesothelioma 5,12,81.
Implications for Future Research
The heterogeneity and relatively scant information of the data demonstrate that well-conducted cause-and-effect relationship between asbestos and MPM and exposure to asbestos history, incidence, and mortality to MPM assessment are required to improve the current evidence support.
Limitations
The main limitations of the conclusions of this review are the relatively scant information provided by the studies that could be obtained about asbestos exposure and incidence and mortality from MPM. The heterogeneity of the studies precluded meta-analysis. This review did not consider papers written in languages different from English, French, and Portuguese, and this might result in not considering relevant studies. Other aspects such as survival, diagnosis, treatment, and compensation were not considered in this review.
Conclusions
There is high evidence to support the relationship between asbestos and MPM, a disease of difficult diagnosis and poor prognosis. There is significant heterogeneity between the small number of identified studies as many are assessed as being of poor methodological quality and at a high risk of bias. All identified studies nevertheless support the relationship between asbestos and MPM, and reinforce the need for well-conducted research, and how research and surveillance are not inseparable. The implementation of National Mesothelioma Surveillance Centres at a global level is mandatory.
Author Contribuitons
C.S., M.A.D., E.S.-L., P.A., and A.S.-U. conceived and designed the study. C.S. and M.A.D. screened the studies and extracted data. C.S. performed data analysis and wrote the first draft of this manuscript. All authors revised the manuscript and approved the final version. C.S. is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and no others meeting the criteria have been omitted.
Data Availability Statement
The study protocol is available at https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021242963. Detailed extracted data on all included and excluded studies are available upon reasonable request to the corresponding author.
The guarantor of this review (C.S.) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained. Dissemination to participants and related patient and public communities: we plan to disseminate the findings and conclusions from this study through scientific conferences.