Introduction and basic concepts of pathogenesis
Photosensitivity is an abnormal cutaneous reaction from light exposure that can be induced or enhanced by topical or systemic drugs, used for therapeutic or diagnostic purposes or in an occupational setting1,2. Drug-induced photosensitivity is a potentially reversible adverse event that occurs when individuals are exposed to a drug and ultraviolet (UV) light or, eventually, visible light, but who tolerate the same amount of light exposure in the absence of the culprit drug1,3.
Drug photosensitivity is certainly underrecognized as it presents under a wide spectrum of clinical patterns with different timelines concerning the relation to drug and light exposure3, and because new culprits are regularly identified4,5.
Photosensitizing agents are chromophores that after absorbing the energy of photons, most commonly from solar radiation, become activated and induce chemical reactions6. Drug photosensitivity is mostly related to UVA (320–400 nm), although some drugs produce reactions upon exposure to UVB radiation (290–320 nm) or even visible light (400–740 nm)6. Only few cases of exclusively UVB-induced drug photosensitivity have been documented4,7.
Acute drug photosensitivity can result from non-specific inflammation–phototoxicity–or a specific immune reaction, mostly T-cell mediated–photoallergy–but other drug-induced or drug-enhanced immune reactions may occur, namely in drug-induced cutaneous lupus erythematosus (LE)8.
Phototoxic reactions can also induce photo- immunosuppression and activate mechanisms involved in photocarcinogenesis and photoaging, responsible for late reactions (premature skin aging, lentigines, actinic keratosis, non-melanoma skin cancer [NMSC], and melanoma)9,10.
Mechanisms of drug-induced photosensitivity
PHOTOTOXICITY
Following photoactivation of the drug present in the skin, the energy of UV photons excites the electrons in the outer orbits of the molecule and, as these electrons come to their ground energy state, the energy lost can produce photochemical modifications in the molecule (isomerization, breaking of double bounds, oxidation) or the energy can be transferred to neighboring molecules, initiating a chain of photochemical reactions. The energy can be directly transferred between two molecules (type I photochemical reaction) or the excited chromophore can react with oxygen, forming free radicals or reactive oxygen species (ROS) that will eventually also activate other molecules (type II photochemical reaction). If cell repair mechanisms (anti-oxidant responses, endonucleases for DNA repair) do not act immediately and control this chain reaction, neighboring molecules relevant for cell survival will be damaged, such as unsaturated lipids of cell membranes, aromatic amino acids of proteins/enzymes and pyrimidine bases of DNA or RNA. Cutaneous cells, namely keratinocytes, will therefore undergo apoptosis (sunburn cells) or necrosis as a result of these phototoxic reactions.
ROS or other abnormal molecules produced in this process will be recognized by intracellular sensors and induce the activation of intracellular signaling pathways (nuclear factor kappa B, mitogen-activated protein kinases, the Nrf-2 antioxidant response element pathway) and inflammasome, generating the secretion of prostaglandins, leukotrienes, interleukins (IL)—1, 6, 8, tumor necrosis factor-alpha (TNF-a), other cytokines and chemokines from keratinocytes and other cutaneous cells. Recruited inflammatory cells cause skin inflammation which becomes clinically apparent within 24–48 h. This is the classical phototoxic reaction that presents mostly as exaggerated sunburn with painful erythema, bullae or epidermal necrosis and on histology with sunburn cells and dermo-epidermal inflammation1,2.
PHOTOALLERGY
In photoallergy, the energy of the UV photon transforms the chromophore into a stable photoproduct (photohapten) or enhances its bonding with an endogenous peptide, forming a photoallergen. By analogy with allergic and photoallergic contact dermatitis, it is suggested that skin antigen-presenting cells become activated and present the new hapten to T cells through human leukocyte antigen (HLA) molecules and costimulatory signals. Sensitized T cells, including memory and effector T cells, mostly Th1 and CD8+ cells, will be activated in a further encounter with the same or a similar chemical and generate a specific T-cell immune reaction, a type IV hypersensitivity reaction11,12. This adaptive T-cell specific immune reaction is mandatory for drug photoallergy, but as in phototoxicity, an initial photochemical reaction may be generated and an innate immune response may create “danger signals” that enhance T-cell sensitization through dendritic cell activation or the expression of adhesion molecules and release of chemokine/cytokine by endothelial cells, fibroblasts, and keratinocytes. Together they promote antigen presentation and the migration of specific effector T cells into the dermis and epidermis, causing the allergic reaction. Therefore, as in allergic contact dermatitis (ACD) where the innate immune skin response to the allergen is well recognized as an important step towards sensitization13, this also probably occurs in drug photoallergy.
PHOTOTOXICITY VS. PHOTOALLERGY
Drug photosensitivity is mainly due to phototoxicity, but some phototoxic chemicals can also induce photoallergic reactions in susceptible individuals14. Phototoxicity and photoallergy are not mutually exclusive and there are some overlapping aspects on pathophysiology and clinical presentations15.
Classically, phototoxicity is more frequent, develops in every individual, as long as a sufficient concentration of the photosensitizer is present in the skin and concomitant exposure to UV in a dose-dependent way. Phototoxicity is somehow predictable, although individual susceptibility certainly exists, may occur after the first contact, and is not associated with flare-ups or cross-reactions in further exposures.
On the other hand, photoallergy develops only in a limited number of individuals, needs previous sensitization but can develop also with chemically similar substances (cross-reactions). It is not strictly dose-dependent and can occur even with low UV doses. Photoallergy presents mostly as pruritic eczema that can spread to non-exposed sites and takes longer to resolve, may become persistent and eventually progress to chronic actinic dermatitis with extreme photosensitivity with no further exposure to the culprit chemical. On histology, there is mainly a dermo-epidermal T-cell infiltrate with epidermal spongiosis and vesicles or a more lichenoid infiltrate. The reaction can usually be reproduced by a photopatch test, particularly in photoallergic contact dermatitis (Table 1)16,17.
Table 1 Main differences between phototoxicity from photoallergy
| Phototoxicity | Photoallergy | |
|---|---|---|
| Frequency | High | Low |
| Latency period/sensitization | No | Yes |
| Doses of UV/photosensitizer | High | Low |
| Cross-reactions | No | Yes |
| Morphology of lesions | Sunburn, polymorphic | Eczema, erythema multiforme |
| Sharp limits | Yes | No |
| Covered areas | Not involved | Possibly involved |
| Resolution | Quick* | May recur, persistent reactors |
| Residual hyperpigmentation | Yes | No |
| Histology | Sunburn cells | Eczema |
| Pathomechanism | DNA/cell damage | Type IV hypersensitivity |
| ROS/inflammation | Photoproduct |
*This relates only to the acute phototoxic reaction, but late effects as photoaging and photodarcinogenesis may also occur.
These two typical polar presentations of drug photosensitivity are easily recognized, but it is not always possible to distinguish between them based on the clinical aspects, histopathology, suspected culprit or results of photopatch or photoprovocation tests.
Except for a few chemicals with no intrinsic phototoxic potential that give rise to stable photoproducts and induce only photoallergy, like piroxicam18, most substances can induce both phototoxic and photoallergic reactions.
Other mechanisms of drug-induced/enhanced photosentitivity
Other immune pathomechanisms may also occur, as some drugs may enhance UV-induced expression of the Ro/SSA antigen on the surface of keratinocytes, interfere with apoptosis or cytokine production and promote photosensitivity and skin lesions in drug-induced subacute cutaneous LE19.
Apart from acute phototoxicity, several phototoxic substances, like psoralens, chlorpromazine fluoroquinolones, and ketoprofen, also enhance chromosomal damage in the presence of UV light, both in vitro and in vivo20-22. These drugs can, therefore, behave as photogenotoxic and photomutagenic. Moreover, DNA aggressions also may cause photo-immunosuppression that further enhances photocarcinogenesis due to the lack of immunosurveillance against cancer cells23. These mechanisms related with fluoroquinolones have recently been reviewed24.
Clinical presentations of drug photosensitivity
Systemic drug photosensitivity presents mainly as exaggerated sunburn or acute eczema on sun-exposed areas, but also as urticaria, lichenoid reactions, telangiectasia, subacute cutaneous LE, bullae, hyperpigmentation, vitiligo-like lesions or NMSC (Table 2)1.
Table 2 Clinical patterns of photosensitivity, mostly involving phototoxicity or photoallergy or other immune-mediated reactions
| Phototoxicity | Immune-mediated reactions |
|---|---|
| Exaggerated “sunburn” | Urticaria |
| Pseudoporphyria | Acute or subacute eczema |
| Photo-onycholysis | Erythema multiform-like |
| Hyperpigmentation | Lichenoid reactions |
| Hypopigmentation (vitiligo-like lesions) | Subacute/chronic lupus erythematosus |
| Telangiectasia | |
| Purpura | |
| Pellagra-like reactions | |
| Actinic keratosis and skin cancer | |
| Accelerated photoaging |
Skin reactions may occur immediately after sun exposure in photosensitivity from vemurafenib, may occur within 1 or 2 days in most phototoxic or photoallergic contact dermatitis or systemic photoallergy, or within several days or weeks in pseudoporphyria, photo-onycholysis or subacute cutaneous LE, or even years, in skin aging and skin cancers enhanced by exposure to photoactive drugs.
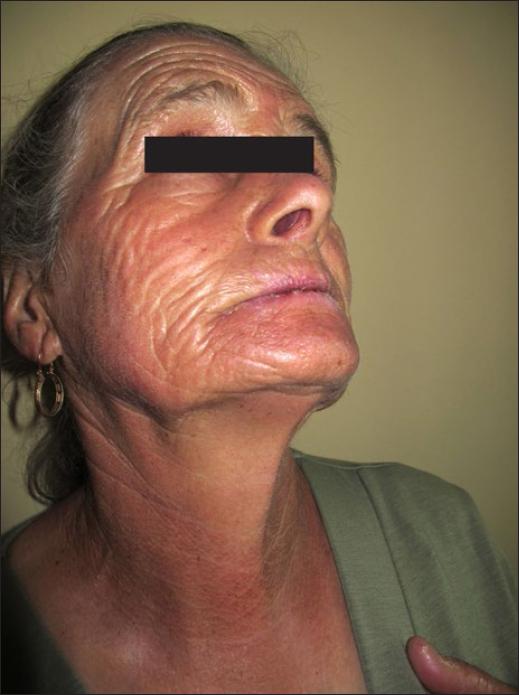
In systemic drug photosensitivity the reaction usually involves the face and forehead, the V shaped area of the neck and upper chest, dorsum of the hands and forearms in a symmetric distribution. Shaded areas of the face (upper eyelids, upper lip, deep wrinkles) are usually spared (Fig. 1) as well as the retroauricular and submandibular areas and other facial areas covered by the beard or hair. Also, large body folds (axillae, groins, finger webs) and areas covered by clothing or accessories (watch strip, shoes) are also usually spared.
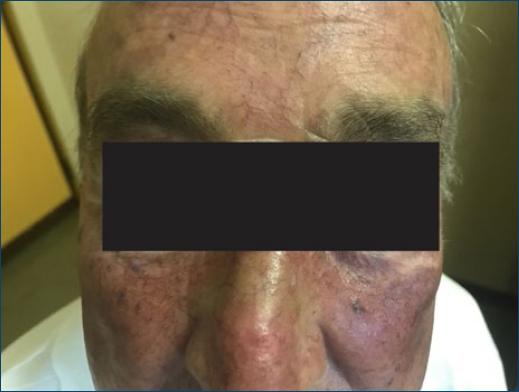
Figure 1 Acute phototoxicity from amiodarone that mimics sunburn and spares the deep facial wrinkles.
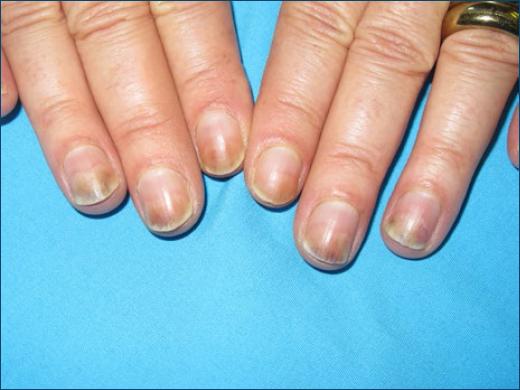
A different distribution of skin lesions can occur when sun exposure is asymmetric, as in car drivers who only expose the left arm/forearm. Occasionally, the lower lip is mainly or almost exclusively involved (Fig. 2), because of higher UV exposure and a thinner corneal layer25,26, or the nails may be involved exclusively, as in photo-onycholysis (Fig. 3)27.
In photoallergic or phototoxic contact dermatitis from topical drugs, lesions occur in the area of concomitant drug application and sun exposure, but distant lesions can occur in areas of accidental contact, as a in a contra-lateral limb (kissing faces of the legs) or in areas of inadvertent spread by the hands or contaminated objects28,29. Cases of connubial dermatitis have been described, mainly for ketoprofen and benzydamine26,30-32. When used as a mouthwash these non-steroidal anti-inflammatory drugs (NSAIDs) induce mostly lip and chin dermatitis26,33.
Some topical drugs applied in large skin areas can be considerably absorbed and induce lesions in a distribution similar to systemic drug photosensitivity.
IMMEDIATE REACTIONS
Immediate urticarial reactions, like photocontact urticaria, have been described with chlorpromazine34 and with 5-aminolevulinic acid used in photodynamic therapy35.
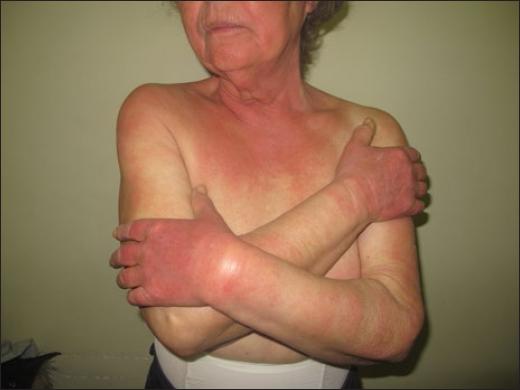
Amiodarone and benoxaprofen induce immediate prickling and burning with transient erythema1. Vemurafenib used as a single drug for metastatic melanoma induces immediate burning upon sun-exposure followed by well-limited painful edema and erythema that persist for a few days occurs in 22–66% of patients8,36, but this reaction is less frequent when vemurafenib is associated with a MEK inhibitor37. A similar pattern has also been described with other BRAF inhibitors and other targeted therapies for cancer, namely the anaplastic lymphoma kinase (ALK) inhibitor brigatinib (Fig. 4)38,39.
ACUTE PHOTOSENSITIVITY
Non-pruritic and sometimes painful sharply limited erythema develops as an exaggerated sunburn in 12–24 h (Fig. 1), with vesicles and/or bullae in more severe forms. It progresses to desquamation and further to residual hyperpigmentation.
In acute drug photoallergy, lesions develop in 12–48 h in sensitized individuals and present mostly as confluent or non-confluent acute or subacute eczematous and pruriginous lesions that may affect also less exposed skin areas. After the culprit drug is stopped lesions usually resolve with no residual pigmentation, but they may progress to lichenification or persistent chronic photosensitivity, sometimes even after drug withdrawal.
In more severe cases of acute photoallergy, erythema-multiforme like lesions occur on photo-exposed and non-exposed areas, as in severe cases of photoallergic contact dermatitis from ketoprofen40 or systemic drug photosensitivity from tocilizumab41, vandetanib42, or statins43. Photo-induced cases of Stevens–Johnson syndrome/toxic epidermal necrolysis have also been associated with drug photosensitivity44.
SUBACUTE PATTERNS OF DRUG PHOTOSENSITIVITY
Drug induced pseudoporphyria develops within weeks to months and presents as chronic skin fragility with flaccid bullae on non-inflamed UV-exposed skin, occasionally progressing to milia. It resembles porphyria cutanea tarda both clinically and on histopathology (bullae below the lamina densa with scarce inflammation), but patients have no inborn error of porphyrin metabolism and no increase of endogenous porphyrins, although some drugs like voriconazole may transiently increase uroporphyrin levels25.
Pseudoporphyria was initially described with nalidixic acid, furosemide, and naproxen, predominantly in children1, but more recently, many other drugs have been associated with this phototoxic reaction: celecoxib45, ciprofloxacin46, voriconazole47, torasemide48, metformin49, finasteride50, and imatinib51.
Photo-onycholysis is a typical pattern of phototoxicity, occurring often as the single manifestation. It presents as a half-moon distal onycholysis of one or several nails (Fig. 3). It appears 2–3 weeks after drug intake and sun exposure and is sometimes preceded by pain in the nail apparatus. It occurs mainly with tetracyclines (demethylchlortetracycline, minocycline, or doxycycline)5, but has also been described with psoralens, fluoroquinolones27, paclitaxel52, and antipsychotic drugs53.
There is no definite explanation for the exclusive nail involvement, but it may be related with less melanin in the nail bed and the nail plate may work as a lens to concentrate UV light27,53.
Drug-induced cutaneous LE is probably underestimated. In a multicentre database analysis of the European Society of Cutaneous Lupus Erythematosus, drug induced cutaneous LE represented 6% among 1002 patients with cutaneous lesions and 13.2% of those with subacute cutaneous LE54. Drug-induced subacute cutaneous LE is usually associated with photosensitivity, mild systemic manifestations and, in >80% of the cases, with positive anti-Ro/SSA auto-antibodies, the hallmark of photosensitivity in LE.
Annular or papulosquamous lesions mimicking the idiopathic form of cutaneous subacute LE usually develop weeks or months after drug exposure (medium of 6 weeks) and can resolve on drug suspension55. Lesions are localized in photoexposed areas (face, neck, upper-chest, and arms), but also in usually UV-shaded areas54. Chronic cutaneous LE with more infiltrated plaques on the face or V of the neck can also be related with drugs.
Subacute cutaneous LE was described initially in association with thiazide diuretics, calcium channel blockers, ACE inhibitors19, and more recently with terbinafine8, the drug associated with the highest odds ratio for this adverse event55. Nowadays there is a long list of other drugs capable of inducing cutaneous LE55, namely proton pump inhibitors56, antiepileptics, TNF-a antagonists55 and the anticancer taxanes, paclitaxel, and docetaxel57.
Dyschromia corresponds to the residual hyper- or hypopigmentation which frequently follows acute phototoxicity. Similarly to the usual UV-induced pigmentary response, IL-1alfa stimulates keratinocytes to produce melanotropins that activate melanocytic pigmentation58. As for hypopigmentation (photoleukomelanoderma), it has been described in flutamide-induced photosensitivity (vitiliginous lesions with sharp limits after the acute reaction)59, and hydrochlorothiazide58.
Dyschromia with solar lentigines and other signs of photoaging have been recently described with voriconazole60 and vandetanib61.
Dyschromia from the accumulation of the photoactive drug or its metabolites in the dermis occurs in a smaller percentage of patients after acute phototoxicity from amiodarone, minocycline, or phenothiazines62. Some patients with lower phototypes also develop a golden-brown, slate gray, or bluish color on sun exposed areas, that persists longer after stopping amiodarone1.
OTHER CLINICAL PATTERNS OF SUBACUTE PHOTOSENSITIVITY
Photo-distributed lichen planus or lichenoid reactions have been reported with several drugs, namely thiazides63, tetracyclines64, quinidine65, capecitabine66, and agents against hepatitis C virus (HCV)–simeprevir and sofosbuvir67. This may represent an individual reaction pattern of photosensitivity as it has been described with two different drugs in the same patient68.
Telangiectasia as a manifestation of photosensitivity has been reported with nifedipine and other calcium channel blockers1, venlafaxine69 and with some cephalosporins5,70. A telangiectatic pattern of photoaging with lesions mainly in the lateral folds of the neck, sparing the shaded skin under the chin, is frequently observed in patients chronically exposed to the sun and photoactive drugs70.
In rare cases, petechial purpura with sharp limits on the transition to the shaded areas was described with ciprofloxacin71.
Pellagra is associated with the prolonged use of isoniazid, that consumes niacin for its metabolism, and pellagroid reactions were reported with the anticancer agents, like 6-mercaptopurine and 5-fluoruracil5 and olanzapine72.
DELAYED AND LATE EFFECTS OF PHOTOSENSITIVITY
Patients chronically exposed to photoactive drugs develop accelerated photoaging, actinic keratosis, and skin cancers, which, at least partially, can be explained by the photogenotoxic effect of some drugs. Voriconazole causing dyschromia, lentigines and actinic keratosis, even in children, is such an example73.
Apart from psoralens, responsible for a dose- dependent increased risk of skin cancers after PUVA therapy74, drugs like naproxen, chlorpromazine, and the fluoroquinolones, particularly lomefloxacin, augment DNA aggression induced in vitro by UV and increase epidermal neoplasia in animals75. In humans, potentially photosensitizing drugs like diuretics and cardiovascular drugs are also being associated with increasing cutaneous pre-cancerous lesions76 and recent reports correlate human short term exposure (weeks/months) to voriconazole or vemurafenib and long exposure to diuretics and anti-hypertensive drugs with an increased risk of developing NMSC and even melanoma5,36,77,78.
Main drugs causing photosensitivity
The catalog of topical and systemic drugs inducing photosensitivity is large and constantly increasing and is not restricted to particular pharmacologic families. Photosensitivity is reported mainly with NSAIDs, antimicrobials (tetracyclines, fluoroquinolones, sulphonamides), psychotropic, cardiovascular, and anti- cancer drugs (Table 3).
Table 3 Main drugs causing photosensitivity
| 1. Nonsteroidal anti-inflammatory drugs (NSAIDs) |
|---|
| Arylpropionic acids: |
| Ketoprofen*,‡, tiaprofenic acid†, suprofen |
| Naproxen¶, ibuprofen, ibuproxam, carprofen‡ |
| Piroxicam*,‡, etofenamate*,‡ |
| Benzydamine‡ |
| Celecoxib¶, diclofenac‡ |
| 2. Antimicrobials (antibiotics, antifungals, antivirals, antimalarials) |
| Tetracyclines† (doxycycline, minocycline, limecycline) |
| Fluoroquinolones** (lomefloxacin†, ciprofloxacin*) |
| Sulphonamides (sulfamethoxazole, dapsone) |
| Isoniazid/pyrazinamide |
| Voriconazole†,**, itraconazole, terbinafine |
| Efavirenz, tenofovir, faldaprevir |
| Quinine, chloroquine, hydroxychloroquine |
| 3. Psychotropic and related drugs |
| Phenotiazines (chlorpromazine‡, thioridazine) |
| Promethazine*,‡, chlorproethazine*,‡ |
| Imipramine, clomipramine |
| Serotonin reuptake inhibitors |
| 4. Cardiovascular drugs |
| Amiodarone†, quinidine |
| Hydrochlorothiazide*,**, furosemide, torsemide |
| Calcium-channel blockers (amlodipine, nifedipine) |
| 5. Anticancer drugs |
| Classical chemotherapy |
| Methotrexate, 6-mercaptopurine, azathioprine, 5-FU |
| Placlitaxel and taxanes |
| Dacarbazine, vinblastine |
| Targeted therapies |
| Vemurafenib** |
| Imatinib¶, sunitinib¶ |
| Erlotinib, vandetanib, pazopanib |
| Brigatinib |
| 6. Miscellaneous drugs |
| Psoralens** |
| Fenofibrate*, simvastatin, atorvastatin |
| Sulfonylureas, sitagliptin, metformin |
| Flutamide, finasteride |
| Pirfenidone |
| Porphyrin analogs for photodynamic therapy |
| Retinoids (isotretinoin) |
| 7.Plants (used as drugs) † |
| Hypericum perforatum (St John's wort) |
| Ruta graveolans (common rue)‡ |
| Kava extracts |
*Mainly photoallergic.
†Mainly phototoxic.
‡Often also from topical exposure or airborne exposure, mainly in occupational settings.
¶Often associated with porphyria cutanea tarda.
**An increase of actinic keratosis, NMSC and, occasionally, melanoma have been related with these drugs.
Nonsteroidal Anti-Inflammatory Drugs
NSAIDs cause photosensitivity when used topically and also after systemic use. Arylpropionic derivatives (benoxaprofen, carprofen, naproxen, suprofen, tiaprofenic acid, ketoprofen, and ibuprofen) have been frequently associated with phototoxicity, with tiaprofenic acid at 5% pet inducing phototoxic reactions in more than half of photopatch tested patients79. Other NSAIDs from this group have been reported to cause photoallergy, occasionally (carprofen and naproxen) or frequently (ketoprofen). Oral naproxen has been associated with photo-distributed erythema multiforme or lichenoid like-lesions, suggesting photoallergy80, but also with pseudoporphyria81. Ketoprofen is the main cause of photoallergic contact dermatitis, although it seldom causes systemic photosensitivity probably due to the low levels reached in the skin after systemic use82.
Ketoprofen used in gel or patches to relieve musculoskeletal pain has caused severe photoallergic reactions83, often with edema, bullae or erythema multiform-like lesions, extending well beyond the area of application84. Reactions may recur on sun exposure with no further drug application, as ketoprofen persists in the epidermis at least for 17 days after application84 and in contaminated objects, namely in clothing after machine washing28.
Photoallergy from piroxicam, frequent 20–30 years ago85,86, occurs in individuals with previous contact allergy to thiomersal87, more precisely to its most frequent sensitizing moiety, thiosalicylic acid88. Actually, upon UVA irradiation, piroxicam decomposes and gives rise to a photoproduct, which is structurally similar to thiosalicylic acid and responsible for photoallergy88.
Photosensitivity from piroxicam usually occurs within 24–48 h after the first drug intake and presents as an acute eczema involving diffusely the whole face or as scattered erythematous papules and vesicles on the face and dorsum of the hands and dyshidrosis86.
Topical diclofenac, used for the treatment of actinic keratosis, has caused allergic and photoallergic contact dermatitis89, sometimes with cross-reactions to aceclofenac90.
Antimicrobials
Oral tetracyclines, doxycycline, and particularly demeclocycline are highly phototoxic and can induce exaggerated sunburn, photo-onycholysis, and pseudoporphyria62. Minocycline, though less phototoxic, can also induce a bluish persistent pigmentation and has caused photo-onycholysis (Fig. 3), like lymecycline91.
Nalidixic acid has caused phototoxicity presenting often as pseudoporphyria92 and the fluoroquinolones with a halogen at C-8 (fleroxacin, lomefloxacin, sparfloxacin, pefloxacin) also induce frequent phototoxic reactions (4–15% of treated patients), whereas this adverse reaction is less frequent with ciprofloxacin, norfloxacin, ofloxacin, and enoxacin91. Hyperpigmentation can occur after lomefloxacine93 and ciprofloxacin have caused pseudoporphyria46, purpura on photoexposed areas71 and photo-induced Stevens–Johnson syndrome44.
Sulfonamides, sulfa-drug analogs (thiazide diuretics, hypoglycemic sulfonylureas, and celecoxib), and dapsone (diaminodiphenylsulfone) have caused photosensitivity within the spectrum of UVB and UVA5,62. This adverse effect is not so frequent with cotrimoxazole, but trimethoprim has also been implicated94.
On rare occasions, other antibiotics have been considered the culprits in photosensitivity, namely the antituberculous drugs isoniazid and pyrazinamide5, and b-lactams ceftazidime and cefotaxime, the latter responsible for telangiectasia on sun-exposed areas70.
The antifungal griseofulvin can cause an exaggerated sunburn-like reaction and aggravate LE95, and terbinafine can also induce subacute LE in patients with anti-Ro antibodies and Rowell syndrome with photo-distribution of skin lesions8.
Among triazole antifungals, itraconazole has seldom been associated with photosensitivity5, but phototoxicity affects more than 40% of patients treated with voriconazole longer than 4–6 months, particularly children73. Cutaneous reactions are dependent on broad UVA, extend to the visible solar spectrum96, and include burning sensation immediately after sun exposure, sunburn like reaction, cheilitis, erosions of the lower lip (Fig. 3), and pseudoporphyria47. On relative short exposures (1–2 years), patients develop accelerated photoaging with solar lentigines and actinic keratosis that soon progress to multifocal invasive squamous cell carcinoma9 or melanoma10. The immunosuppressed state of most patients on long-term voriconazole may enhance cutaneous carcinogenesis, but skin cancers related with voriconazole are distinct and more aggressive than those described in organ-transplanted or other immunosuppressed patients97.
Antivirals used for human immunodeficiency virus (HIV) or HCV infection have been associated with photosensitivity confirmed by photopatch or photoprovocation tests5. Efavirenz induced mostly photo-distributed papulosquamous annular lesions within a few days or weeks of treatment98 tenofovir induced a severe photo-distributed reaction with further generalization interpreted as a photoallergy99 and the combination of faldaprevir and deleobuvir caused photosensitivity in more than a quarter of patients involved in controlled clinical trials100.
The old antimalarials quinine and quinidine have frequently caused photoallergic and phototoxic reactions101. Chloroquine and hydroxychloroquine, mostly used in dermatology to prevent photosensitivity in cutaneous LE and polymorphic light eruption, paradoxically also cause photosensitivity102 and a similar reaction has also been described with the combination of atovaquone and proguanil used in malaria prophylaxis103.
Psychotropic drugs
Phenothiazines used systemically as antipsychotics (chlorpromazine, thioridazine, and flupenthixol) are typically phototoxic and cause sunburn, bullous and lichenoid eruptions, and photo-distributed slate-gray hyperpigmentation on the long term5. Olanzapine has caused several cases of photosensitivity53,72, as well clozapine, haloperidol, and riperidone5.
Photoallergy occurs frequently after contact with phenothiazines, namely with creams containing promethazine or isothipendyl chlorhydrate used as topical antipruritics85 chlorproethazine cream used for muscle pain104 or chlorpromazine manipulated by caregivers105.
Among antidepressants, tricyclic drugs imipramine and clomipramine, as well as the newer selective serotonin reuptake inhibitors, fluoxetine, paroxetine, fluvoxamine, venlafaxine, and sertraline, have been proven as occasional causes of photosensitivity, similar to the anxiolytics alprazolam and chlordiazepoxide5.
Cardiovascular drugs
Soon after its introduction in the market, the diuretic hydrochlorothiazide was recognized as a cause of photosensitivity, often proven by positive photopatch tests. Exaggerated sunburn, photo-distributed eczema, lichenoid reactions, dyschromia, persistent photosensitivity106 and more recently increase of actinic keratosis and NMSC have been related with its wide use76.
The antiarrhythmic amiodarone frequently causes phototoxicity (7–50% of patients) presenting as burning/tingling sensation on sun-exposure, followed by erythema, eczema and a bluish-gray hyperpigmentation in sun-exposed areas due to the dermal accumulation of drug metabolites107.
Other cardiovascular drugs have been associated with photo-induced reactions, like amlodipine and nifedipine (telangiectasia)108,109, diltiazem (lichenoid reaction with hyperpigmentation)110, furosemide/torsemide (pseudoporphyria, subacute LE, photo-onycholysis) and, very occasionally, angiotensin conversing enzyme inhibitors (lisinopril, enalapril, and ramipril), candesartan and the b-blockers atenolol and bisoprolol4.
Anticancer drugs
Many classical anticancer drugs and particularly new targeted therapies have been increasingly associated with photo-induced cutaneous lesions38. Examples of classical drugs include the antimetabolites, methotrexate (sunburn recall reaction), 5-fluoruracil and related capecitabine and tegafur (sunburn, lichenoid and eczematous reactions, hyperpigmentation), 6-mercapto-purine and azathioprine (pellagra-like reactions and photocarcinogenesis)4, dacarbazine5, paclitaxel and other taxanes (erythema multiforme, photo-onycholysis, drug-induced LE)111 and vinblastine5.
Among new targeted therapies, photosensitivity has been described with imatinib (several cases of pseudoporphyria)51, sunitinib (pseudoporphyria)112, brigatinib (sunburn like reaction)39, erlotinib113 (also with acne-like reactions predominating in sun-exposed areas), crisotinib5, pazopanib (phototoxicity and hyperpigmentation)114, vandetanib (phototoxic and several photoallergic reactions with erythema multiforme-like aspects, confirmed by photopatch tests)61,114, and, particularly, with vemurafenib. When used as a single therapy in metastatic melanoma up to two thirds of patients develop vemurafenib photosensitivity in the spectrum of UVA presenting as immediate burning and painful sensation and a sharply demarcated erythema and edema that appear still during UV irradiation, resembling solar urticaria, but erythema and edema persist for a few days77.
Other drugs
Despite its recognized phototoxic potential, the anti-fibrotic agent pirfenidone was released for the treatment of interstitial lung disease and has caused phototoxic reactions with photoleukomelanoderma115 and photoallergy with lichenoid reactions and positive photopatch or photoprovocation tests11 6.
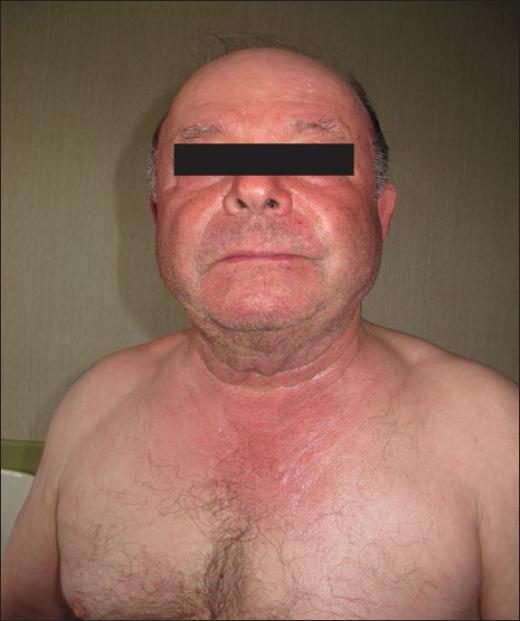
Fenofibrate can cause photoallergy with lichenoid and eczematous reactions (Fig. 5) due to its benzophenone structure and often exhibits cross-reactions with ketoprofen117. Statins, both simvastatin and atorvastatin, have also induced photosensitivity with reduced sensitivity to UVA4.
Retinoids, namely isotretinoin increases UV sensitivity, and very occasionally photosensitivity has been described with antidiabetics (glibenclamide and sitagliptin), the anticonvulsant carbamazepine4, flutamide118, and finasteride50 and even with amoxicillin119. Therefore, all suspected drugs have to be considered and photopatch or photoprovocation tests should be performed to establish a correct diagnosis.
Moreover, photosensitivity can be due to “folk” medicines, mostly based on plant extracts rich in furocoumarins, like “home-made” infusions of St. John's wort (Hypericum perforatum L.) used to treat depression120 or Ruta graveolans infusions applied topically to relieve pain in fibromyalgia121.
Diagnostic Procedures in Drug Photosensitivity
In suspected drug photosensitivity, it is very important to question the onset of drug use and the relation with sun exposure, with a particular emphasis into the amount and type of sun light received, including through a window glass which allows permeation of UVA mostly involved in drug photosensitivity. In typical cases of photosensitivity after exposure to a known photosensitizer and resolution on drug withdrawal, no additional tests are required.
Photopatch tests are indicated mainly for confirming the etiology of photoallergic contact dermatitis, but they can also be useful in the study of systemic drug photosensitivity122. For this procedure, allergens in patch test chambers are applied in duplicate on the back, followed by skin irradiation of one of the sets of allergens at day 1 or day 2 with 5 J/cm2 of UVA, whereas the other set is shielded from light. Readings should be performed immediately after irradiation and also 48 and/or 72 h thereafter, comparing the irradiated vs. non-irradiated area of the back. Positive reactions both in the irradiated and non-irradiated sites mean contact allergy, that may be photoaggravated if the reaction is 1+ more in the irradiated site. A photopatch test is positive when erythema and papules covering the whole test area are observed only in the irradiated side123,124. If the reaction is mainly erythema and edema, without pruritus, exclusively limited to the test chamber area, with very sharp limits, begins shortly after irradiation, reaches its highest intensity by 24 h and regresses by 48/72 h (decrescendo reaction) with hyperpigmentation, it suggests a phototoxic reaction. A pruritic erythema with vesicles, diffuse limits extending beyond the chamber limit, increasing in intensity until 48/72 h after irradiation (crescendo reaction), suggests photoallergy125. Often these patterns are not so typical and the difficulties previously referred in the interpretation of clinical cases also occur in the interpretation of the photopatch tests.
The recommended European baseline photopatch test series includes many topical and systemic drugs, namely ketoprofen, etofenamate, piroxicam, benzydamine, and also piketoprofen, dexketoprofen, ibuprofen, diclofenac, fenofibrate, and chlorpromazine in the extended series89. In the absence of standardized commercial allergens, which are really few for the study of systemic drug photosensitivity, drugs can be photopatch tested after the powder of the commercial drug is incorporated in petrolatum or in water, as recommended for the study of other non-immediate cutaneous drug eruptions. Photopatch tests are often positive in photoallergy to piroxicam86, ketoprofen126, fenofibrate117, and also occasionally with hydrochlorothiazide106, lomefloxacin15, pirfenidone116, and vandetanib61.
Photoprovocation is also helpful to confirm the culprit in systemic drug photosensitivity4. For photoprovocation small areas of the back/buttocks are irradiated with increasing UV doses and, if possible, different UV-wavelengths. Readings are performed immediately or within 24/72 h. Reaction to very low UV doses for the phototype or a significant difference in the minimal erythema dose (after UVA or UVB irradiation) between phototests performed while the patient is exposed to the drug or after its withdrawal is considered positive.
Photoprovocation using a monochromator allows identification of the precise UV-wavelength responsible for the photosensitivity in order to avoid it in future drug exposures.
With highly phototoxic drugs, both photopatch and photoprovocation tests can be positive in the majority of tested individuals, therefore, they are not particularly useful for confirming the etiology of a phototoxic reaction, but they can disclose a hidden photoallergy.
Conclusions
Phototoxic, photoallergic, and overlapping photosensitive reactions are still a frequent problem. They have highly polymorphic clinical presentations with different time courses concerning exposure to the drug and to the sun, and therefore the diagnosis can be difficult. Main culprits depend on geographic areas and over time, mostly related to prescription habits. The dermatologist must be highly alert to search for a possible involvement of a drug in a photosensitive patient and try to confirm its contribution to photosensitivity. A correct questionnaire should be conducted, and although not so important in phototoxic cases, complementary tests including photopatch and photoprovocation tests may contribute to the final etiologic diagnosis. This is important in order to allow an adequate patient advice concerning further eviction of the photosensitizer and related chemicals, which, apart from acute symptoms, are being increasingly associated with accelerated photoaging and enhancement of skin cancers.
What does this study add?
Clinical manifestations of drug phototosensitivity are polymporphic and, apart from exaggerated sunburn and acute eczema, can also present as erythema multiforme, pseudoporphyria, photo-onycholysis, dyschromia, lichenoid reaction, telangiectasia or purpura on sun-exposed areas and subacute LE or enhance photoaging and risk of NMSC (or melanoma).
Main topical drugs causing photosensitivity are the NSAIDs, particularly ketoprofen and systemic drugs also include NSAIDs, fluoroquinolones, tetracyclines, thiazides, and phenothiazines, but a few new drugs have been described as culprits.
Photopatch testing, indicated mainly for the study of photoallergic contact dermatitis, can also be useful in systemic drug photosensitivity.