Introduction
Solid organ transplant recipients (SOTR) require chronic immunosuppressive therapy, making them particularly predisposed to opportunistic skin infections and skin cancer1. Given the significant burden of cutaneous disease in these patients, their clinical management team should optimally include dermatologists working in the context of a transplant dermatology clinic. Dermatological care should start before the organ transplant procedure takes place, since some features may help in predicting and consequently preventing complications. Follow-up should be regular as some relevant skin problems are time-dependent and may occur several years after transplantation2.
Skin infections may be caused by viral, bacterial, and fungal organisms and are a major source of morbidity and mortality in SOTRs3. About 70% of severe skin infections appear during the first 3 months after transplantation, but they can occur at any time2. The risk changes according to the treatment schemes and different periods after transplantation have their own characteristic infection pattern3.
Fungi are the most frequent cause of skin infection in SOTRs, with a cumulative incidence of 50% in Western countries. Fungal infections develop mainly during the first 2 years after organ transplantation, especially in older patients and liver transplant recipients2. There is a large spectrum of diseases, ranging from superficial (e.g., dermatophytes) to deeper and more severe infections (with agents such as Alternaria, Aspergillus, Cryptococcus, and Histoplasma)2.
It is important for care providers of SOTRs to recognize and appropriately manage fungal cutaneous infections to improve patient outcomes4.
In the literature, there is more data on systemic fungal infection than on superficial fungal infections, which are mainly of interest to dermatologists3. Herein, we perform a narrative review of primary cutaneous fungal infections in SOTR. Disseminated fungal infections are beyond the scope of this article and will not be discussed5.
Effect of immunosuppressive drugs
Immune factors that may affect the development of fungal infection are mainly neutrophil deficiency, abnormal blood count, myeloperoxidase deficiency, dysfunction of T lymphocytes, mononuclear phagocytes, and damage to the reticuloendothelial system5. Other important factors are the pathogenicity of the fungal cells, their enzymatic activity, the properties of epithelial cells of the skin and mucosae, and the coexistence of local and systemic factors that cause increased proliferation of saprophytic fungi.
Immunosuppressive drugs used in these patients have different mechanisms of action. Cyclosporine and tacrolimus are calcineurin inhibitors and inhibit the production of interleukin-2 (IL-2), leading to impairment of T cell-mediated cytotoxicity and, indirectly, affecting B cell growth and antibody production, by suppression of T cell-derived growth factors necessary for these functions6. Azathioprine is a thiopurine and acts by inhibiting purine synthesis, affecting the activity of T cells and to a lesser extent B cells, affecting mainly the cells that are dividing. Glucocorticoids impair the migration of neutrophils, monocytes, and macrophages and decrease the activity of cytotoxic T lymphocytes3. Mycophenolate mofetil is an inhibitor of inosine-50-monophosphate dehydrogenase, a critical enzyme for the synthesis of the novo guanosine nucleotides, expressed in activated T and B cells, resulting in lymphocyte inhibition7. Sirolimus and everolimus are mammalian targets of rapamycin (mTOR) inhibitors and disrupt cytokine signaling that promotes lymphocyte growth and differentiation.
These immunosuppressors impair cell-mediated immunity, decrease the number of antigen-presenting Langerhans cells in the epidermis, and (particularly the corticosteroids) disrupt the stratum corneum, increasing the susceptibility to fungal infections8-10.
Drug interaction awareness is paramount in the management of fungal infections in SOTR. Cyclosporine and tacrolimus are known substrates of CYP3A11, while triazole compounds, such as itraconazole and Fluconazole, are potent inhibitors of the CYP3A4 enzyme subfamily, potentially leading to an increase in tacrolimus and cyclosporine blood concentrations. Therefore, upon initiation of oral azole therapy, these immunosuppressant drug levels must be monitored and adjusted after consultation with the nephrologist. Reduction of the dose of the tacrolimus and cyclosporin may be necessary, to avoid the risk of side effects, particularly nephrotoxicity12,13.
Cutaneous fungal infections in SOTRs
Fungi can be divided into groups based on their growth patterns. They generally display one out of two growth patterns, yeast-like or filamentous. Dimorphic fungi can exist in two forms: as molds (with septate hyphae and conidia) in nature and as other forms (usually yeasts) in living tissue. Yeast genera include Candida, Malassezia, Cryptococcus, and Trichosporon. Filamentous fungi include Dermatophytes, Aspergillus, Mucor, Rhizopus, Rhizomucor, Fusarium, and Dematiaceous Fungi (Curvularia, Alternaria). Dimorphic fungi include Coccidioides, Histoplasma, and Blastomyces4.
The authors opted to divide the cutaneous fungal infections into three groups: (1) “superficial,” (2) “subcutaneous” and (3) “deep” infections, according to the dept of infection that these organisms most frequently cause (Fig. 1). It is important to note that in the “deep” infections subchapter, which includes organisms that most frequently cause systemic infections which will not be addressed in this article, as we will focus on primary cutaneous infections.
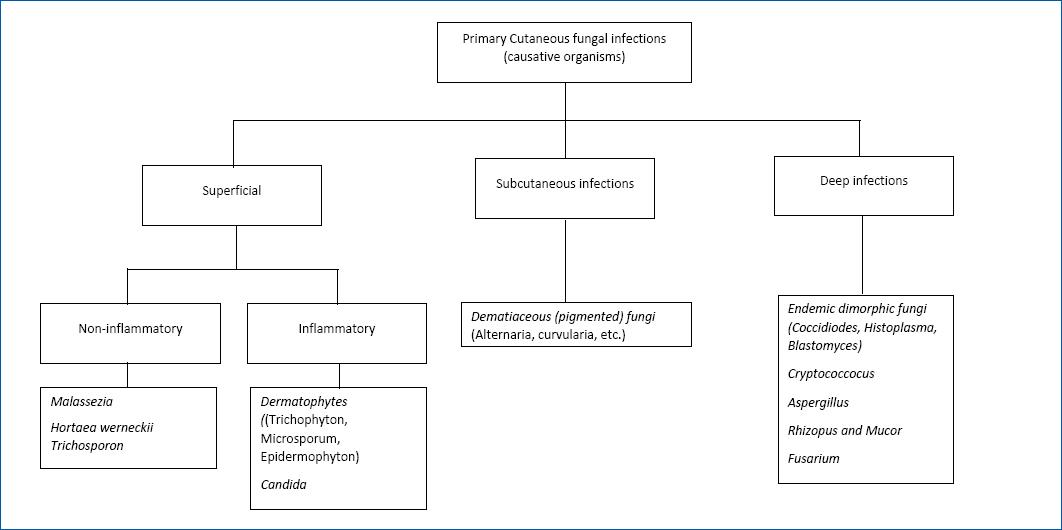
Figure 1 Algorithm showing different fungi causing primary cutaneous infections in solid organ transplanted recipients. Note that in the non-inflammatory agents group, mild inflammation may occur in some cases.
SUPERFICIAL FUNGAL INFECTIONS
Superficial mycoses are the most common fungal infections occurring in SOTRs and the less severe ones. They usually affect the stratum corneum and skin appendages, such as the hair and nails2.
We can divide these infections into non-inflammatory, such as tinea versicolor, tinea nigra, and piedra, and inflammatory, mostly caused by Dermatophytes and Candida.
MALASSEZIA
Malassezia furfur is a yeast that colonizes healthy human skin but is also capable of causing infection4. Some studies have found Malassezia colonization on the upper back to occur twice as frequently in SOTRs compared to healthy controls, while others have not found a statistically significant difference3,4.
Malassezia infection may lead to the development of pityriasis versicolor (PV) or pityrosporum folliculitis (PF) (Fig. 2). PV presents as numerous oval macules, patches, or plaques with a fine-scale that may be hypopigmented (due to inhibition of melanin production by dicarboxylic acids that result from the metabolism of skin surface lipids by the yeast) or hyperpigmented. These lesions are commonly distributed over the chest, back, and upper arms. PF is associated with sebaceous inflammation owing to Malassezia furfur converting triglycerides into free fatty acids14. PF appears as an acneiform pruritic eruption with erythematous papules and pustules on the upper back, chest, scalp, neck, and arms. Patients may also present with a generalized dermatosis.
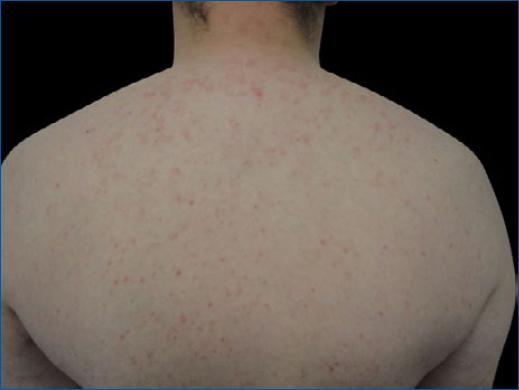
Figure 2 Malassezia folliculitis, with papular and follicular pruritic lesions on the trunk in a SOTR.
Diagnosis of Malassezia infection is usually clinical but can be confirmed by direct exam (examining skin scrapings under a microscope with potassium hydroxide [KOH] preparation). A fungal culture is not used when diagnosing Malassezia spp. as these fungi, due to their lipid requirement, cannot grow on conventional media. A biopsy is reserved for cases with a high degree of doubt.
Treatment of PF or PV infections involves the use of topical antifungal creams and shampoos, including imidazoles, ciclopirox, and selenium disulfide. Systemic azole therapy should be used if there is resistance to topical treatment or when large areas are involved. Examples of the latter include fluconazole (200 mg daily for 5–7 days, 200–300 mg weekly for 2–3 weeks; or 400 mg once) or itraconazole (200 mg daily for 5–7 days)15. Caution on drug interactions is mandatory. Recurrence is frequent and maintenance therapy may be of use.
TRICHOSPORONOSIS
Trichosporon spp. are the yeasts that are ubiquitous in subtropical warm temperate climates, commonly found in the soil, water, and human mucosa. Trichosporon species include T. inkin, T. cutaneum, T.ovoides, and T. loubier. Even in immunocompetent hosts, they can proliferate in hair shafts causing white piedra, which refers to tan or brown nodules on terminal hair shafts distributed on hair throughout the body, usually sparing the scalp. T. asahii may cause disseminated infection in the immunosuppressed, particularly in neutropenic patients4.
Treating white piedra involves shaving hair within the infected region and applying a 2% ketoconazole shampoo daily until the infection is resolved. Persistent cases of white piedra are managed with 100 mg/day oral itraconazole in addition to topical therapy4.
DERMATOPHYTES
Dermatophytosis are common cutaneous infections in SOTRs caused by three genera of filamentous fungi: Microsporum, Epidermophyton, and Trichophyton. These occur in up to 5.6% of SOTR and are transmitted by other affected individuals or acquired by contact with the soil or affected animals such as dogs, cats, horses, birds, or other animals16. Similar to the immunocompetent population, the most common dermatophyte infection in SOTR is Trichophyton rubrum, a predominantly anthrophilic fungus linked with the development of tinea cruris, tinea corporis, tinea unguium, and tinea pedis (Fig. 3).
Dermatophytes metabolize keratin, and their infections are normally limited to keratinized structures, such as the stratum corneum, hair shafts and nails. Occasionally, especially in immunocompromised hosts, the dermatophyte infects the hair follicle and extends into the deep dermis, forming a granuloma around it, causing pink or red perifollicular papules, abscesses, plaques and nodules, in what is called a Majocchi's granuloma or nodular granulomatous perifolliculitis. The lower extremities are more likely to show disease due to the tendency for trauma and onychomycosis in that region15. Further dissemination to the internal organs is exceedingly rare, due to the fungus requirement for keratin17,18.
Tinea capitis is a common dermatophyte infection in SOTRs that presents with scaly macules or patches and sometimes alopecia. In the immunocompromised, tinea infections tend to be diffuse, non-specific, and recur frequently4.
Onychomycosis is characterized by nail thickening, yellowing, and subungual debris. This condition usually afflicts toenails and is associated with concurrent tinea pedis infection. In the SOTR population, the involvement of multiple toes or fingernails is common. Proximal subungual onychomycosis, defined by extending infection from the proximal nail fold to the ventral nail plate is virtually pathognomonic of immunosuppression19.
It is important to note that dermatophytosis, even in SOTRs, is a benign infection.
For patients with suspected dermatophytosis, initial evaluation using a KOH preparation and culture of skin scrapings is recommended. Superficial infections should first be managed with a course of topical therapy, with agents such as azoles, allylamines, ciclopirox, and butenafine20,21. Effective treatment of onychomycosis, tinea capitis, and superficial infections refractory to topical therapy require systemic antifungal agents. In Majocchi's granuloma, the first line of therapy is terbinafine 100 mg daily, which should be continued for approximately 12 weeks.12,15
As previously explained, drug interactions must also be taken into account, and tacrolimus and cyclosporine blood levels must be carefully monitored upon initiation of oral azole therapy12.
CANDIDA
Candidiasis is a fungal infection caused by yeasts of the genus Candida, encompassing over 20 species, the most common of which is C. albicans. Other species include C. tropicalis, and rarely C. krusei, C. Toluropsis, C. glabrata, C. parapsilosis, and C. pseudotropicalis. These yeasts normally reside in the intestinal tract and can be found on mucous membranes and the skin without causing infection, but their overgrowth can be associated with disease2. Systemic Candida infection is a serious condition that may present with cutaneous findings, but it is beyond the scope of this paper.
Regarding localized infections, oral thrush is the most common fungal infection in SOTRs within the first post-transplant year, afflicting up to 64% of transplant recipients23 (Fig. 4). It presents with thick, curd-like white papules and plaques upon an erythematous base that can be removed with rubbing. Oral candidiasis can also present as angular cheilitis (perlèche) that affects the corners of the mouth, leading to cracking, fissuring, and crusting with underlying erythema, and as stomatitis, that presents with a bright, erythematous, glossy palate. Patients on corticosteroid or antibacterial therapy, diabetics, and denture users are at increased risk of oral candidiasis24. In oral infections, clotrimazole (10 mg five times daily) is superior to nystatin (100,000 units/ml suspension: 4–6 ml four times daily). Severe cases can be treated with oral fluconazole (200 mg on day 1, and then 100–200 mg/day until 7–14 days after clinical resolution).
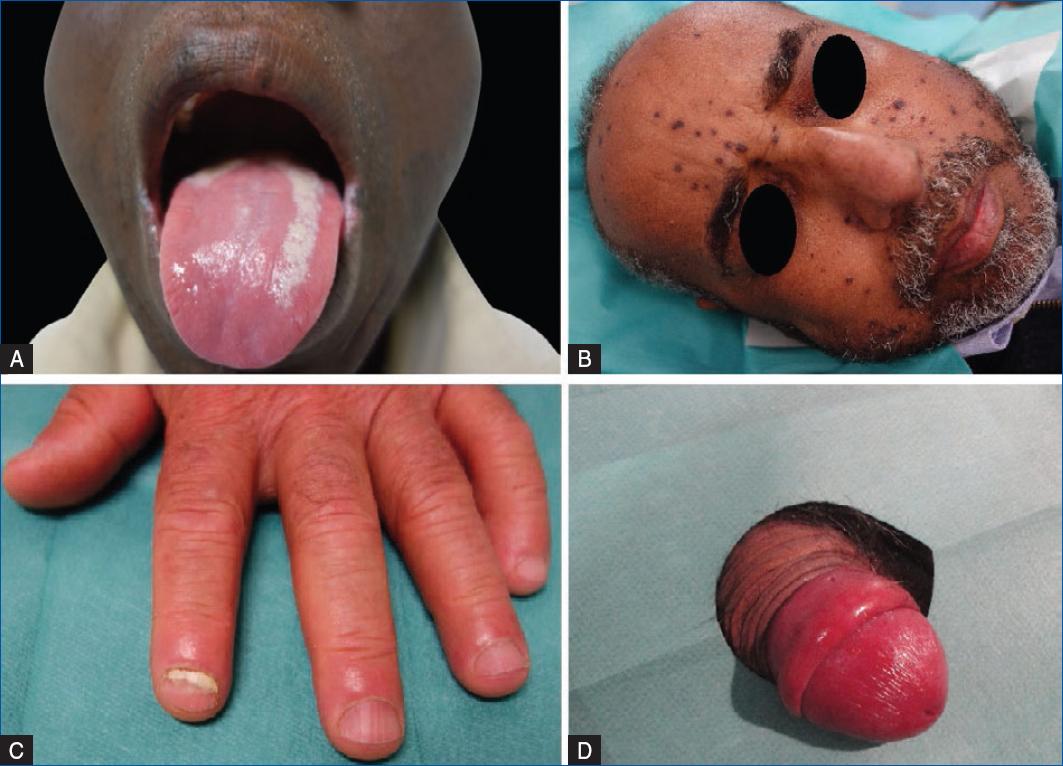
Figure 4 Candida infections in SOTRs. A: oral thrush. B: violaceous papulonodules on the face of a patient with disseminated candidiasis. C: proximal subungual onychomycosis. D: balanitis.
Candidiasis of the nail presents as perionychia with edema and discoloration at the lateral proximal nail fold, along with occasional pus formation. Superficial Candida skin infections are treated with topical antifungals such as imidazoles, allylamines, or nystatin25. Short courses of systemic agents should be used when topical therapy fails (e.g., fluconazole 50–100 mg daily for 14 days, fluconazole 150 mg/weekly for 2–4 weeks or itraconazole 200 mg twice daily for 14 days)15.
Universal prophylaxis for Candida is not routinely used in renal, heart or lung transplant recipients; however, prophylactic fluconazole therapy is recommended for 1–2 weeks' post-transplant in pancreatic transplant recipients26. Additionally, high-risk liver transplant recipients on prophylactic liposomal amphotericin-B or echinocandins for 3–4 weeks' post-transplant had reduced rates of invasive candidiasis27.
SUBCUTANEOUS INFECTIONS
Deeper skin infections involving the dermis and subcutis are less common (1%) but more serious than superficial infections. Their incidence is higher during the first months after transplantation, with 65% of infections occurring in the first 2 years after transplant, most commonly after traumatic inoculation28. The main risk factor is frequent contact with soil or plants28. This group encompasses Dematiaceous fungi (brown pigmented) and includes a heterogenous group of diseases such as chromoblastomycosis, mycetoma, sporotrichosis, and lobomycosis29.
PHAEOHYPHOMYCOSIS
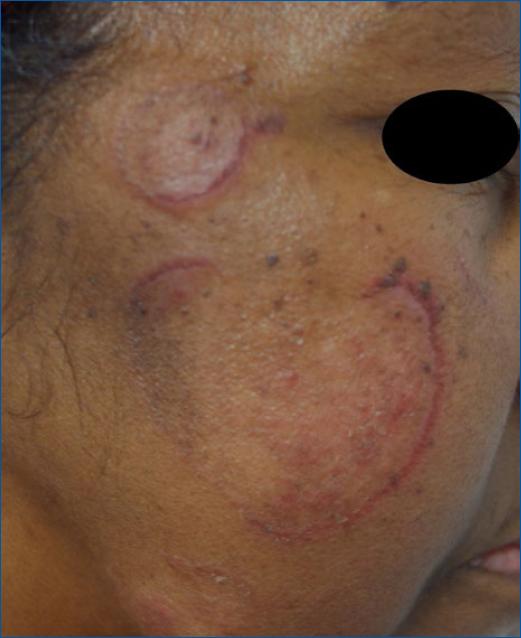
Phaeohyphomycosis include infections with Alternaria spp, Exophiala spp and Phialophora spp. Patients with a history of gardening or cutaneous trauma with soil exposure are particularly prone to Alternaria infection, although these are typically benign. They most frequently present on the extremities as a subcutaneous asymptomatic nodule with a central cyst that may evolve into an abscess. Phaeohyphomycosis due to Alternaria spp. in SOTRs can present as individual or multiple pustules, plaques, papulonodules, and ulcers30 (Fig. 5).

Figure 5 Alternaria spp infections in SOTR; A: cutaneous nodules in the leg. B: nodule on the dorsal surface of finger.
For well-limited lesions surgery alone has been effective. Cryotherapy, laser, and photodynamic therapy have also been used successfully. Oral antifungals, mainly azoles, have been widely used as co-adjunctive therapies in immunocompromised patients as to prevent dissemination. Multiple subcutaneous nodules should be treated with systemic antifungal agents, such as itraconazole 400 mg/day or voriconazole 400 mg/day31.
CHROMOBLASTOMYCOSIS
Chromoblastomycosis is a relatively rare infection, that is endemic to particular regions of the world, and classically presents as an expanding verrucous plaque on the lower, or occasionally upper extremity, sometimes being difficult to clinically differentiate from squamous cell carcinoma or Kaposi's sarcoma32.
The diagnosis is confirmed by the culture of biopsy samples and histopathology. Biopsy of a chromoblastomycosis lesion shows thick-walled spheric spores that resemble copper pennies, known as Medlar bodies, which confirm the diagnosis4.
Surgical excision or cryotherapy may be sufficient to treat this infection, including in SOTRs, but the addition of systemic therapy with itraconazole (200–400 mg/day), posaconazole (800 mg/day) or terbinafine (500–1000 mg) improves cure rates33,34.
MYCETOMA
Mycotic mycetoma or eumycetoma is a chronic fungal infection of the skin and the subcutaneous tissue, more commonly seen in men and caused by different fungi, including Madurella mycetomatis, especially frequent in Africa, Madurella grisea, common in South America; and Pseudallescheria boydii (Scedosporium apiospermum), more frequently seen in the USA, but also reported in Europe. It usually presents as a single painless lump, mostly on the foot, growing slowly until it ulcerates and discharges a purulent exudate with grains29. The proper treatment of mycetoma depends mainly on accurate diagnosis. The current diagnostic tools include imaging techniques such as radiography, ultrasonography, CT scan, MRI, molecular techniques such as PCR, serodiagnostic tests such as ELISA, counterimmuno-electrophoresis, as well the classical grain culture and histopathological diagnosis35.
First line treatment of eumycetoma includes surgical removal followed by ketoconazole 400–800 mg/day or itraconazole 400 mg/day for extended periods of time, ranging from a few months to several years, with a mean duration of 12 months35.
SPOROTRICHOSIS
Sporotrichosis, also called rose gardener disease, is caused by the fungus Sporothrix schenckii. Cutaneous sporotrichosis is the most common variant and occurs in individuals who have handled contaminated plant materials. Pulmonary sporotrichosis is rare, occurring after inhalation of fungal spores from the environment. Disseminated sporotrichosis usually affects the immunosuppressed.
The cutaneous form is characterized by the presence of nodules distributed along the lymphatic vessels, starting from the area surrounding the primary lesion. These nodules gradually grow and tend to become pustular and ulcerate. The diagnosis of sporotrichosis is made by histopathological examination, which reveals a granulomatous infection, and by culture of infected tissue. First-line treatment is itraconazole (100–200 mg/day). Fluconazole (400 mg/day) is less effective and should only be used if the patient cannot tolerate itraconazole36. Saturated potassium iodide oral solution three times a day is another treatment option. Patients with severe forms of sporotrichosis are usually treated with amphotericin B (e.g., 3–5 mg/kg of lipid formulation) and flucytosine (50–150 mg/kg/day given four times daily for 6–12 weeks)2,37.
DEEP INFECTIONS
Classically, the group of deep fungal infections includes organisms that frequently cause systemic fungal infections, however, as previously explained, in this article we will only cover the primary cutaneous diseases caused by these agents.
This group is frequently divided into two subgroups: (1) “true fungal pathogens” virulent species that can cause infection in a host with normal immune status, and (2) “opportunistic agents” that mainly affect immunocompromised patients. Most of these organisms are dimorphic fungi.
CRYPTOCOCCOSIS
Cryptococcus spp., particularly C. neoformans, are opportunistic facultative intracellular yeasts that cause mild pulmonary disease in the immunocompetent and may cause severe disseminated disease in the immunocompromised4. However, there are a few reports of localized primary cutaneous cryptococcal disease in SOTRs38. Cutaneous manifestations of cryptococcosis present as non-specific polymorphic lesions including papules, pustules, vesicles, nonhealing ulcers, cellulitis, subcutaneous nodules, ecchymoses, gummas, abscesses, and granulomata. Lesions suspicious of cryptococcal infection must be biopsied and cultured to confirm the disease. Treatment of primary cutaneous cryptococcal lesions includes fluconazole (200–400 mg/day) and itraconazole (200–600 mg/day), guided by clinical response, but usually for at least 3 months. For patients with more severe disease, treatment with amphotericin B (0.5–1 mg/kg/d) may be necessary for 6–10 weeks39.
It is important for care providers to educate patients on sources of Cryptococcus infections, such as pigeon excrement4,17.
ASPERGILLOSIS
Cutaneous manifestations of Aspergillosis mostly occur due to systemic infection, however cases of primary cutaneous aspergillosis have been reported. Two of the most clinically relevant species are Aspergillus fumigatus, the most common in disseminated disease, and Aspergillus flavus, more likely to cause primary cutaneous aspergillosis. Most commonly, aspergillosis occurs in the form of chronic respiratory infections, but in immunocompromised patients, soft tissue infections have been described, usually presenting as erythematous plaques or nodules and cellulitis-like lesions. They usually occur due to direct inoculation, e.g., contaminated devices such as catheters or IV devices, and present with ecthyma and necrotic lesions17,40.
A definitive diagnosis of aspergillosis is made via biopsy and culture. Systemic voriconazole (6 mg/kg IV every 12 h for 1 day, followed by 4 mg/kg IV every 12 h; oral therapy can be used at 200–300 mg every 12 h) is recommended as primary therapy. In cases of aspergillosis in burns or massive soft tissue wounds, in addition to anti-fugal therapy, surgical debridement is recommended41. Alternative agents include liposomal amphotericin B (L-AmB), posaconazole, itraconazole, or an echinocandin, with the clinical response guiding the duration of therapy17,42. As previously explained, it is important to reduce calcineurin-inhibitor doses when combining these agents with azoles, so as to prevent immunosuppressant-associated toxicity43.
MUCORMYCOSIS
Mucormycosis is a fungal infection mostly associated with the Rhizopus and Mucor species.4. Risk factors include malignant hematological disease with or without stem cell transplantation, prolonged and severe neutropenia, poorly controlled diabetes mellitus with or without diabetic ketoacidosis, iron overload, major trauma, prolonged use of corticosteroids, illicit intravenous drug use, neonatal prematurity, and malnourishment44.
The clinical hallmark of invasive mucormycosis is tissue necrosis resulting from angioinvasion and subsequent thrombosis. In most cases, the infection is rapidly progressive and results in death unless underlying risk factors (e.g., metabolic acidosis) are corrected and aggressive treatment with antifungal agents and surgical excision is started. Based on its clinical presentation and anatomic site, invasive mucormycosis is classified as one of the following six major clinical forms: (1) rhinocerebral, (2) pulmonary, (3) cutaneous, (4) gastrointestinal, (5) disseminated, and (6) uncommon forms, such as endocarditis, osteomyelitis, peritonitis, and renal infection45.
Cutaneous mucormycosis results from direct inoculation of fungal spores in the skin, most frequently due to trauma17. It usually presents as a necrotic eschar accompanied by surrounding erythema and induration. However, a non-specific erythematous macule, although small and apparently insignificant, may be the cutaneous manifestation of disseminated disease in an immunosuppressed patient45.
Diagnosis involves identification of the organism in tissue, confirmation with culture, and/or polymerase chain reaction (PCR). Treatment involves urgent surgical debridement, correction of metabolic abnormalities, reduction of immunosuppression, and antifungal therapy. The antifungal of choice is deoxycolate amphotericin B (d-AmB); however, it is often substituted by lipid formulations because of their better safety profile, being less nephrotoxic, allowing longer treatment periods with higher doses. In immunocompromised patients, the recommended doses for d-AmB are 1–1.5 mg/kg/day, for L-AmB, 5–10 mg/kg/day, and for amphotericin B lipid complex 5 mg/kg/day. In order to increase the survival rate, treatment must be started in the first 5 days after clinical diagnosis. Some authors recommend continuing AmB until clinical and radiological resolution, others recommend 6–8 weeks46,47. Posaconazole is recommended as a second-line treatment for patients with refractory disease or intolerance to AmB or for those who need prolonged treatment maintenance. The suggested dose is 400 mg twice daily, and in most reported cases therapy is provided for several months48. Prognosis remains poor45.
FUSARIOSIS
Fusarium species are hyalohyphomycetes (non-pigmented/hyaline fungi) that are ubiquitous, being widely distributed in the soil, air, and plants. In humans, Fusarium species may cause disease that is localized, focally invasive, or disseminated. This pathogen generally affects immunocompromised individuals, although there are rare reports of infections in immunocompetents49.
Table 1 Summary of the recommended treatments for each fungal cutaneous infection. In case of systemic dissemination, other schemes may apply. In general, when lesions are localized, surgical excision/debridement may be an option
| Malassezia infection15 | Topical clotrimazole 1%, selenium sulfide 1% or 2.5%, ketoconazole 1% or 2%, zinc pyrithione or ciclopirox olamine twice daily for 14 days. Severe or recalcitrant cases can be treated with fluconazole (200 mg daily for 5–7 days, 200–300 mg weekly for 2–3 weeks; or 400 mg once) or itraconazole (200 mg daily for 5–7 days). |
| Trichosporonosis | Shave hair; 2% ketoconazole shampoo daily until infection is resolved. In persistent cases, 100 mg/day oral itraconazole in addition to topical therapy. |
| Dermatophytes | Superficial infections can be managed with topical azoles, allylamines, ciclopirox and butenafine. If hair |
| infection15,22 | involvement, deep infection or refractory to topical therapy, terbinafine should be used as first line (in Majocchi's granuloma, treatment should be continued for approximately 12 weeks). Azoles may be used but require caution due to drug interactions. |
| Candidiasis15 | Oral infections: clotrimazole (10 mg five times daily) is superior to Nystatin (100,000 units/ml suspension: 4–6 ml four times daily). Severe or recalcitrant cases can be treated with oral fluconazole (200 mg on day 1, and then 100–200 mg/day until 7–14 days after clinical resolution). Intertrigo: topical antifungals such as imidazoles, allylamines or nystatin twice daily for 1–2 weeks. Severe or recalcitrant cases can be treated with fluconazole (50–100 mg daily for 14 days or 150 mg weekly for 2–4 weeks) or itraconazole (200 mg twice daily for 14 days). Onychomycosis: Topical or systemic treatment (e.g., Itraconazole 200 mg for one week per month for 3–6 months). |
| Phaeohyphomycosis31 | Surgical excision or debridement is recommended whenever feasible and may be sufficient for isolated cutaneous disease; Itraconazole 400 mg/day or voriconazole 400 mg/day are first line agents. |
| Chromoblastomycosis33,34 | Surgical excision or cryotherapy may be sufficient treatment, but addition of systemic therapy with itraconazole (200–400 mg/day), posoconazole (800 mg/day) and terbinafine (500–1000 mg) improves cure rates. |
| Mycetoma | Combination of surgical excision and debridement and ketoconazole 400–800 mg/day or itraconazole 400 mg/ day. Other agents include voriconazole (400 mg/day) and Posaconazole (800 mg/day). |
| Sporotrichosis35 | First-line treatment is itraconazole (100–200 mg/day). Fluconazole (400 mg/day) is less effective and should only be used if the patient cannot tolerate itraconazole. Severe cases may require amphotericin B (e.g., 3–5mg/kg of lipid formulation) + flucytosine (50–150mg/kg/day given four times daily for 6–12 weeks). |
| Cryptococcosis2,36,37 | Long courses Fluconazole (200–400mg/d) or Itraconazole (200–400 mg/d) (duration from 2 weeks to 6 months, depending on clinical response). For patients with more severe disease, treatment with amphotericin B (0.5–1 mg/kg/d) may be necessary for 6–10 weeks. |
| Aspergillosis39 | Voriconazole (6 mg/kg IV every 12 h for 1 d, followed by 4 mg/kg IV every 12 h; oral therapy can be used at 200–300 mg every 12 h) is recommended as primary therapy. Alternative agents include Liposomal amphotericin B, posaconazole, itraconazole, or an echinocandin. |
| Mucormycosis46,47,48 | Surgical excision/debridement is recommended for all cutaneous infections. Liposomal amphotericin B (5–10 mg/ kg/day) is the treatment of choice. Posaconazole (400 mg bid) is recommended as second-line treatment for patients with refractory disease or intolerance to amphotericin B or for those who need prolonged treatment maintenance. |
| Fusariosis49,50,52 | Onychomycosis: Itraconazole 200–400 mg/day or pulse therapy. A second commonly used drug is terbinafine at doses of 250–500 mg/d, sometimes combined with topical ciclopirox and amorolfine lacquer, or with keratolytics, such as urea. Cutaneous infection: surgical excision or debridement is recommended whenever feasible. Long treatments of Itraconazole 200 mg/day. Other agents include terbinafine, amphotericin B, voriconazole, posaconazole, and ravuconazole. |
*Oral azole therapy in SOTR requires monitorization of cyclosporine and tacrolimus levels and possible dose adjustment after consultation with a nephrologist.
The localized disease most often presents as onychomycosis and keratitis. Fusarium-onychomycosis is clinically indistinguishable from onychomycosis by dermatophytes and may present as distal subungual, proximal subungual, and total dystrophic infection. Associated paronychia is not uncommon. Appropriate treatment is recommended because of the risk of dissemination from a local nail lesion. Direct examination with KOH reveals thin, irregular hyphae, and confirmation of etiology by repeated culture and absence of growth of dermatophytes and yeasts. The therapeutic outcome of Fusarium onychomycosis, particularly in subungual cases, is variable. Treatment options include itraconazole 200–400 mg/day or pulse therapy. A second commonly used drug is terbinafine at doses of 250–500 mg/day, sometimes combined with topical ciclopirox and amorolfine lacquer, or with a keratolytic, such as urea. Fusarium paronychia can be treated with the drugs mentioned or with topical treatment, preferably occlusive to achieve better concentrations in skin and nails49,50. The clinical response is slow.
Other presentations of localized cutaneous fusariosis include cellulitis or intertrigo51. All skin lesions suspicious of fusariosis in immunocompromised patients should be biopsied and sent for histopathology and microbiological studies. The skin can be an important and early clue to diagnosis since cutaneous lesions may be observed at an early stage of the disease. Typical skin lesions may be painful red or violaceous nodules, the center of which often becomes ulcerated and covered by a black eschar. Multiple necrotizing lesions are often observed on the trunk and the extremities. First-line treatment of deeper cutaneous infections include surgical excision, voriconazole 6 mg/kg IV every 12 h for two doses followed by 4 mg/kg IV every 12 h, or L-AmB 3–5 mg/kg IV once daily52. Lipid-based amphotericin B preparations exhibit fewer side effects when compared with amphotericin B deoxycholate and should be favored52.
Conclusion
SOTRs are at high risk for cutaneous adverse events. General impairment of the immune system predisposes to the development of acute or chronic fungal cutaneous infection20.
When treating fungal infections in these patients side effects and drug interactions with immunosuppressive therapy must always be considered.
Cutaneous manifestations of fungal infections in SOTRs can vary in onset, presentation, severity, and prognosis. Most present in the same low-risk manner as in non-immunocompromised hosts, however, these patients are at a higher risk for systemic dissemination.
It is important to be aware of the diversity of fungi that can cause cutaneous disease and to promptly obtain the necessary biopsies or tissue culture, allowing early diagnosis and treatment, as to limit associated morbidity and mortality.