Introduction
Over recent years, the number of cases of onychomycosis caused by non-dermatophytic filamentous fungi (NDF) has increased, with a high prevalence of Scytalidium and Fusarium species1-3. As well as dermatophytes, Scytalidium and Fusarium are keratinase-producing fungi and, due to this ability, they become primary nail pathogens–unlike other NDF that do not have this ability and, therefore, are often considered secondary pathogens or contaminants4-6.
Scytalidium is considered an endemic pathogen in hot-humid climate areas. The most known species to cause onychomycosis are S. dimidiatum and S. hyalinum. Both are filamentous fungi identified by the branching, septate hyphae with arthroconidia arranged in the chain and aerial mycelium of grayish to black color, which differentiate them from dematiaceous and hyaline fungi, respectively1,4,7.
Fusarium is a hialohyphomycete with optimum temperature growth between 25 and 30°C. Micromorphological characteristics of this fungi reveal canoe-shaped septate macroconidia and the aspect of the colony shows a felty mycelium with different colors8,10. The main species that cause onychomycosis are F. solani and F. oxysporum and they can be differentiated by their micromorphological features4,8,9. F. solani microconidia are larger, more oval, and have a thicker wall, however, it is difficult to make this distinction only through microscopy examination10.
For the correct microbial identification of these pathogens, it is essential to carry out a culture test. The direct examination does not allow mycologic identification, since the reproductive structures can only be identified within the culture by the micromorphological aspects11. Besides the difficulties of fungal identification, these species have an increased resistance to the available antifungal drugs for dermatological use8.
Onychomycosis caused by NDF has predisposing factors that include tropical and subtropical climate, mechanical trauma, prolonged use of closed shoes, hyperhidrosis, family history, and immunosuppression, mainly in HIV patients2,5. In most cases, NDF causes superficial infections, however, immunocompromised patients can develop a systemic disease5,7,8. Within this framework, the correct diagnosis carried out with direct examination, culture test and fungal antibiogram are crucial to an effective therapy–avoiding antifungal resistance and toxic effects5.
The aim of this study is to demonstrate the main features of the structures of Fusarium spp. and Scytalidium spp. important for a reliable mycological diagnosis. Using data obtained from the mycology sector of a clinical analysis laboratory located in Porto Alegre, Rio Grande do Sul, Brazil, epidemiologic aspects, such as prevalence, age, and sex of the patients were evaluated.
Methods
Data collected from the mycological examination obtained from a clinical analysis laboratory located in Porto Alegre/RS was evaluated through a transversal retrospective 5-year analysis from January 2017 to December 2021. The frequency of fungi diagnosed by mycological culture was analyzed and divided into two samples: onychomycosis caused by Scytalidium spp. and onychomycosis caused by Fusarium spp. Each sample was subdivided into macro- and micromorphology, and the age and sex of the patients, site of infection, and year of the examination were also collected.
The prevalence of Fusarium spp and Scytalidium spp infection was determined using all culture methods available in the clinical analysis laboratory, with or without direct examination, dividing the laboratory test reports into onychomycosis and another dermatomycosis (such as skin or scalp lesions, and secretions). Exclusion criteria were (i) laboratory test reports that revealed fungal contamination; and (ii) direct examinations that detected Malassezia spp. since the correct identification of this fungi requires a positive culture in a specific growth medium11,12.
The nail samples were collected using sterile pliers and sent to the mycology sector of the laboratory, followed by direct examination and culture tests. For the direct examination, part of the sample was processed with potassium hydroxide 20% for clearing the specimens and enhancing the microscopic observation of fungal structures. For fungal identification, fungal culture tests were used. The samples were inoculated in Potato Dextrose Agar and Sabouraud Dextrose Agar with Chloramphenicol and incubated for a period of 21 days at 25°C.
Results
During the study period, 7250 mycological examinations (direct examination and/or fungal culture test) were performed. Out of the 7250 laboratory test reports, 3426 fungal culture tests were solicited for diagnosis of cutaneous lesions, 2479 for onychomycosis, and 839 for other dermatomycoses. A total of 1595 cases of onychomycosis and 383 other dermatomycoses were confirmed. Among the 1595 confirmed cases of onychomycosis, 881 (55.24%) were positive for dermatophytes, 345 (21.63%) for yeasts, and 369 (23.13%) for NDF. As seen in Table 1, prevalence rates for dermatophytes were 35.5%, 13.9% for yeasts, and 14.9% for the NDF.
Table 1 Results of culture tests performed for the diagnosis of onychomycosis in the last 5 years
| Year | ||||||
|---|---|---|---|---|---|---|
| Diagnosis | 2017 | 2018 | 2019 | 2020 | 2021 | Total (%) |
| Dermatophytes | 139 | 161 | 148 | 255 | 178 | 881 (35.5) |
| Yeasts | 65 | 144 | 91 | 24 | 21 | 345 (13.9) |
| NDF | 81 | 100 | 93 | 48 | 47 | 369 (14.9) |
| Negatives | 100 | 166 | 226 | 120 | 272 | 884 (35.7) |
| Total | 385 | 571 | 558 | 447 | 518 | 2479 (100) |
NDF: non-dermatophytic filamentous fungi; (%) percentage calculated in relation to the number of culture tests performed for onychomycosis (2479).
Among all samples identifying NDF group, 280 laboratory test reports were positive for Scytalidium spp. and Fusarium spp., 249 in cases of onychomycosis, representing about 10% of the analyzed samples from onychomycosis (2479). Out of 249, 175 (7%) confirmed Fusarium spp. and 74 (3%) Scytalidium spp. A relevant feature was the negative direct examination in 199 of the 249 samples, representing 79.9% of the total. Table 1 shows the distribution of findings for each year.
Onychomycosis cases caused by most NDF decreased in the last years–exhibiting their highest number in 2018. However, Fusarium spp. showed a gradual increase, with 40 cases in the year 2020, when it reached its peak representing 83% of the onychomycosis caused by NDF. In 2021, Fusarium spp. reports decreased. For Scytalidium spp., the incidence of the pathogen has decreased by more than 50% since the study was started (Table 2). Concerning another dermatomycosis, NDF was found in 31 cases, eight cases in 2017 and 2018, six cases in 2019, three in 2020, and six in 2021.
Table 2 Number of positive mycological tests for Scytalidium spp. and Fusarium spp. in onychomycosis from January 2017 to December 2021
| Year | ||||||
|---|---|---|---|---|---|---|
| 2017 | 2018 | 2019 | 2020 | 2021 | Total | |
| Scytalidium spp. | 25 | 19 | 16 | 7 | 7 | 74 |
| Fusarium spp. | 37 | 33 | 38 | 40 | 27 | 175 |
| Total (%) | 62 (24.9) | 52 (20.9) | 54 (21.7) | 47 (18.9) | 34 (13.6) | 249 |
(%) Percentage of the total number of positive reports of onychomycosis caused by Scytalidium spp. and Fusarium spp. among the 249 cases by NDF.
Among the 74 cases of onychomycoses caused by Scytalidium spp., 63.5% were females and the most affected age group was between 30 and 39 years (17.6%), whereas for the 175 cases caused by Fusarium spp., the most affected group were women (74.9%) aged between 50 and 59 years (18.9%) (Table 3).
Table 3 A number of cases of onychomycosis by Scytalidium spp. and Fusarium spp. separated by sex and age of the patients
| Scytalidium spp. (Total = 74) | Fusarium spp. (Total = 175) | |||
|---|---|---|---|---|
| Age | Women (%) | Men (%) | Women (%) | Men (%) |
| 0-9 | 0 (0) | 0 (0) | 1 (0.6) | 0 (0) |
| 10-19 | 0 (0) | 0 (0) | 2 (1.1) | 1 (0.6) |
| 20-29 | 0 (0) | 6 (8.1) | 16 (9.1) | 6 (3.4) |
| 30-39 | 13 (17.6) | 9 (12.2) | 22 (12.6) | 11 (6.3) |
| 40-49 | 8 (10.8) | 6 (8.1) | 27 (15.4) | 10 (5.7) |
| 50-59 | 8 (10.8) | 5 (6.8) | 33 (18.9) | 7 (4) |
| 60-69 | 12 (16.2) | 1 (1.3) | 18 (10.3) | 5 (2.9) |
| 70-79 | 3 (4.05) | 0 (0) | 5 (2.9) | 3 (1.7) |
| 80-89 | 3 (4.05) | 0 (0) | 7 (4) | 1 (0.6) |
| Total (%) | 47 (63.5) | 27 (36.5) | 131 (74.9) | 44 (25.1) |
(%) Percentage refers to the number of positive reports of onychomycosis caused by Scytalidium spp. (74) and Fusarium spp. (175).
The site of the affected nail was also evaluated. For Scytalidium spp., 79.8% of the cases affected the toenails, 2.7% the fingernails, and 1.3% both were infected and 13.5% of the samples were related to another dermatomycosis. For Fusarium spp., toenails also represented 78.3% of the cases, and fingernails 9.1%. Both fingernails and toenails were infected by Fusarium spp. in 2.3% of the cases and 6.9% of the samples were collected from other dermatomycoses. As seen in Table 4, the prevalence of affected toenails is significantly higher compared to other sites of infection for both pathogens.
Table 4 Comparison between the numbers of cases relating to the characteristics found in the mycological reports
| Samples of Scytalidium spp. (74) n (%) | Samples of Fusarium spp. (175) n (%) | Total samples (249) n (%) | |
|---|---|---|---|
| Positive direct examination | 18 (24.3) | 31 (17.7) | 49 (19.7) |
| Negative direct examination | 56 (75.7) | 143 (81.7) | 199 (79.9) |
| Direct examination not performed | 0 (0) | 1 (0.6) | 1 (0.4) |
| Dermatophyte-associated | 6 (8.1) | 6 (3.4) | 12 (4.8) |
| Site of infection | |||
| Hallux | 17 (23) | 35 (20) | 51 (20.5) |
| Affected hallux (right or left) | 17 (23) | 61(34.9) | 78 (31.3) |
| Thumbs | 0 (0) | 2 (1.1) | 2 (0.8) |
| Toes | 25 (33.8) | 41 (23.4) | 66 (26.5) |
| Fingers | 2 (2.7) | 14 (8) | 16 (6.4) |
| Fingernails + toenails | 1 (1.3) | 4 (2.3) | 5 (2) |
| Nail + skin infection | 10 (13.5) | 12 (6.9) | 22 (8.8) |
| Nails (no site recorded) | 3 (4) | 6 (3.4) | 9 (3.6) |
(%) Percentage refers to the number of positive reports of onychomycosis caused by Scytalidium spp. (74), Fusarium spp. (175) over the total of samples (249).
In 12 cases (4.8%) these NDF fungi were considered contaminants since there was a coinfection by dermatophytes.
Morphological characteristics of Fusarium spp. include clear surface colonies with variations in reverse (white, beige, salmon, pink, violet, orange, red and brown), filamentous, with a cotton-like aspect, as shown in Fig. 1.
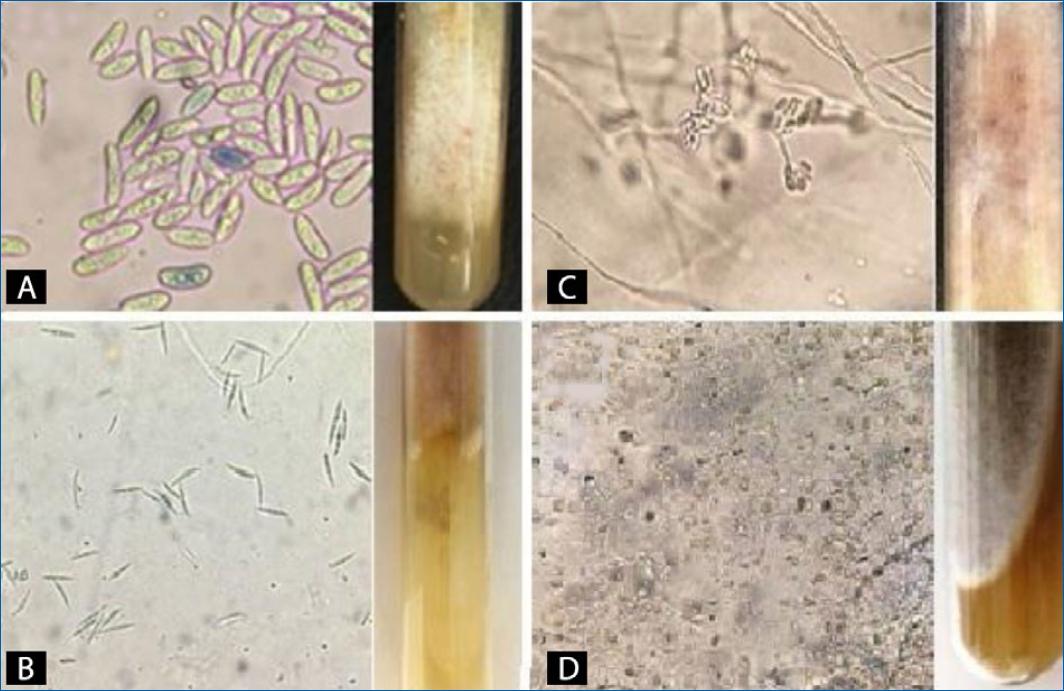
Figure 1 Fusarium spp. A: elongated and ovoid macroconidia, some microconidia with the same shape as macroconidia, presenting pink refringence and white colony with a white reverse and yellow and pink pigmentations. B: fusiform macroconidia and beige colony with brown reverse, filamentous appearance. C: microconidia grouped in “false heads” and white colony with salmon reverse. D: many oval and pyriform microconidia, some fusiform macroconidia and loose and chained chlamydoconidia, a white colony with dark brown reverse. (Source: Mont’ Serrat Laboratory).
Morphological characteristics of Scytalidium spp. reveal dark colonies, ranging from brown to black or gray (Fig 2A to C), with the exception of Scytalidium hyalinum which may have a white colony (Fig. 2D).
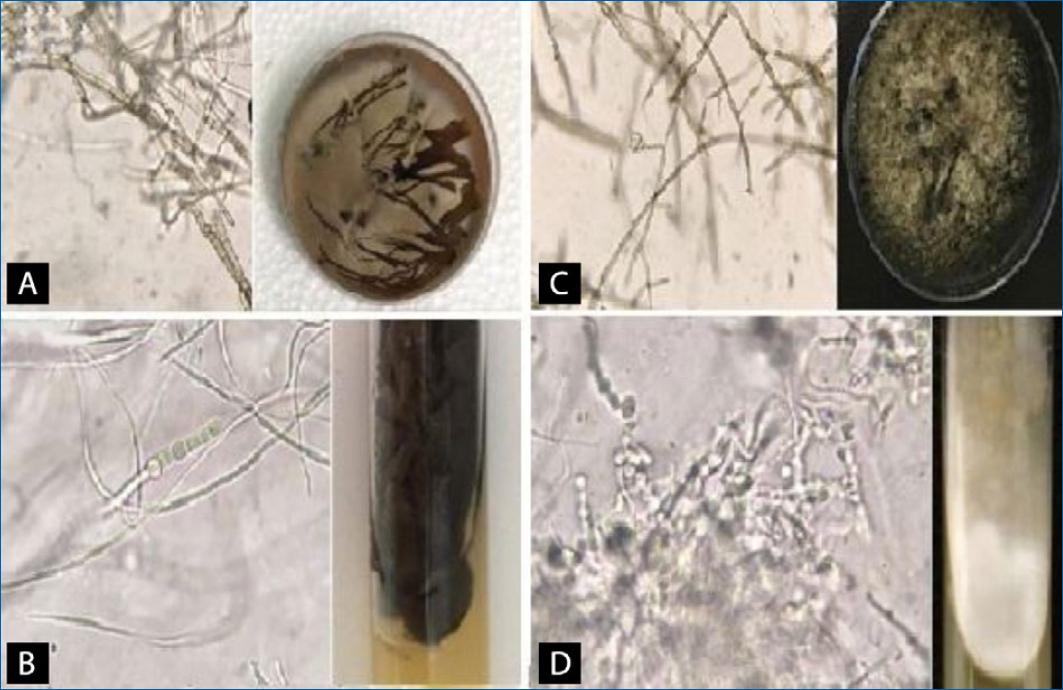
Figure 2 Scytalidium spp. A: broad demaceous and septate hyphae, some thin and hyaline hyphae, arthroconidia in the chain of oblong shape, and grayish-brown colony with dark-brown reverse, characteristic of Scytalidium dimidiatum. B: two types of hyphae, dematiaceous and hyaline, chain of plump arthroconidia generated from the hypha and black colony with yellow reverse. C: long chains of cylindrical arthroconidia with or without septum and gray colony with black reverse. D: arthroconidia in round chains and hyaline hyphae, a white colony with yellowish reverse, characteristic of the species Scytalidium hyalinum. (Source: Mont’ Serrat Laboratory).
Discussion
Scientific literature indicates that dermatophytes are the most frequent agents of onychomycosis, representing about 80-90% of the cases4-6. Furthermore, yeasts account for about 5-17%, while the frequency of NDF varies from 2 to 22%2,13,14. In addition to the pathogens of interest in this study, many species belong to the NDF group, such as Curvularia spp., Acremonium spp., Scopulariopsis brevicaulis, and Aspergillus spp.4-6. In this study, the frequency of NDF and yeasts detected in onychomycosis (respectively 23 and 22%) is increased compared to literature2,6, whereas dermatophytes were less frequently observed (55%). Fusarium spp. and Scytalidium spp. represent 11% and 4.63%, respectively.
A study in Rio de Janeiro, Brazil, showed that 11.86% of onychomycosis was caused by NDF. Besides, Cursi et al.3 found that 4.86% of the nail infections were caused by Scytalidium spp., representing 40.9% of NDF group. Females were the most affected (2:1) and were aged between 40 and 60 years. On the other hand, our study revealed that despite the similar prevalence (3%), the sample (74) represented 20% of the NDF, also having a predominance of females (2:1). However, the age group was younger (30-39 years). Both studies demonstrate that NDF nail infection is not a rare situation3.
A recent study2, also performed with Fusarium spp. and Scytalidium spp., exhibited a high prevalence of these pathogens in adult women. The authors correlated Fusarium spp. infection, with pedicure history, as women often go for pedicures and also with their tendency to have more contact with the ground, since they wear open shoes more often. These may be explanations for such findings, as we also found in the sample (175) a prevalence of women (3:1) aged 50-59 years. Moreover, elderly people tend to have a higher incidence of onychomycosis because they have less nail growth and more circulation problems that disrupt nail plate morphology and also have more frequent underlying diseases2.
Onychomycosis represents up to 50% of nail diseases15 and can be classified according to their location and mode of invasion. The most common forms are distal lateral subungual onychomycosis (DLSO), proximal subungual onychomycosis, superficial onychomycosis, and total dystrophic onychomycosis5,6. The manifestation of DLSO, which predominates in the feet, is the most common form of involvement by Scytalidium and Fusarium. This infection caused by these NDF is indistinguishable from DLSO caused by dermatophyte fungi.
Gupta et al.16 reviews is frequently used as a reference for the diagnosis of onychomycosis caused by NDF. They point out that it is necessary to meet at least three of the following features to be considered a true positive result: identification by direct examination, isolation of NDF in fungal culture, repeated isolation in culture, exclusion of dermatophytes, inoculum counting, and histopathology2-4,16.
NDF found in samples collected for the diagnosis of onychomycosis could indicate primary pathogens, contaminants, transient colonization, secondary colonizers, and persistent secondary colonizers. This implies that the nail can be initially infected by a dermatophyte and then by a NDF, even after antifungal therapy–which often generates selective pressure causing resistant fungi to continue colonizing the nail5. Therefore, the association with dermatophytes found in 4.8% of the cases in this study does not necessarily mean contamination and exclusion of the pathogenic effect of the NDF, which may be classified as a secondary pathogen.
Many studies2,3,5,15,16 prioritize fungal identification during the direct microscopic examination. However, it is known that several factors interfere with the result, such as previous use of antifungal drugs, the technical skill of the operator, and mainly preanalytical questions such as poor hygiene prior to sample collection, poor investigation of the use of antifungal drugs and inadequate sample collection in addition to the fact that the procedure must be performed in a manner compatible with the type of pathology6,11. Quatrin et al.11 in a comparative study revealed that the positive predictive values and negative predictive values of direct mycological examination are 78% and 44%, respectively, compared to a fungal culture which is 97% and 91%, reinforcing the importance of performing mycological cultures in all cases. Actually, in the present study, about 80% of the samples with a positive culture were negative on the direct microscopic examination.
As mentioned above, one of the criteria for validating the diagnosis of onychomycosis caused by NDF is repeated isolation in fungal culture. However, there is great difficulty in getting patients to return for repeat exams, both for logistical and financial reasons. Therefore, it is difficult to apply this method in clinical laboratories. The inoculum counting is also considered one of the criteria, however, for Scytalidium and Fusarium, filamentous fungi that form a “carpet” over the entire plate as shown in Figure 1 and 2, it is not possible to perform this counting. Still, on the validation parameters, histopathology can also be used for evaluation, and despite supplying the deficiencies of the direct examination, it requires more steps in the preparation of the slide and a pathologist to analyze5,15 which is not part of the routine of the clinical analysis laboratory of this study.
Furthermore, it is important to consider that Fusarium and Scytalidium are mainly parasites of vegetation, being facultative parasites of humans. Mimicking the ideal environment for the growth of these fungi is extremely difficult. In this way, attention should be paid to the structures that grow in the cultural media, especially in cases of cultural examinations with the growth of a single microorganism considering that contamination usually occurs by the growth of more than one fungal type in the same culture.
Regarding the microscopic and macroscopic structural features of Fusarium spp., most samples revealed hyaline hyphae and microconidia in different shapes–oval, ellipsoid, piriform, and fusiform, loosely arranged or grouped together in “false heads” (Fig. 1C). In this case, operator experience is crucial, as it can be confused with Acremonium spp., which forms clusters of fusiform microconidia. Chlamydoconidia, structures typical of fungal resistance17, were also visualized (Fig. 1D). The color of the mycelium ranged from white to beige, with the reverse of different colors (pink, violet, orange, brown and red). For a correct diagnosis, it is necessary that macroconidia are visualized since they are specific to each species. Fusarium spp. macroconidia are larger structures than their microconidia and have the shape of a banana, a canoe, or a half-moon (Fig. 1).
On the other hand, Scytalidium spp. showed two hyphae shapes. One type has hyaline, smooth, and narrow hyphae and the other has wide, dematiaceous, and septate hyphae, as shown in Fig. 2. The main feature is the arthroconidia long rows, formed from the hyphae. These arthroconidia have a plump or cylindrical shape and the mycelium has a flaky texture. Some colonies are dark (black, gray or brown) with a yellow to dark brown reverse and others are white colonies with a yellow reverse (Fig. 2).
During the analysis of the data (Table 1), it was noticed that in 2020 and 2021 there was a sudden decrease in positive reports for NDF and despite the number of mycological cultural tests performed in the period was slightly lower than in 2018 and 2019, this large drop in cases is not well clarified. However, these years coincide with the period of the SARS-CoV-2 coronavirus pandemic, and hygiene and health care guidelines were strongly recommended, which may have led to a decrease in cases of these environmental and opportunistic fungi and explain the reduction of positive reports for Scytalidium and Fusarium.
Conclusion
Onychomycosis caused by NDF is a significant problem even though it decreased in the last 5 years, due to a decrease in the cases due to Scytalidium spp. However, Fusarium spp. reports remain high compared to the overall picture. Adult women are the most affected group and the feet are the most affected site of infection for both pathogens. For the diagnosis, it is essential to perform the fungal culture. For correct identification, technical skills and scientific knowledge are crucial to identify the particular characteristics of Fusarium spp. (fusiform micro and macroconidia) and Scytalidium spp. (oblong-shaped chain arthroconidia).














