Introduction
Problems with the development of cancer after the use of sartans (angiotensin receptor blockers) date back to 2010 when their first use in patients with hypertension was associated with a negligible increased risk of developing various (or any type) of cancer1. Twelve years later, the same authors performed a meta-analysis with statistically “much more mature” considerations and concluded that the use of sartans for a longer period (over 2.5/3 years), as well as at the maximum daily dose, is clearly associated with a significant risk of developing cancer2. However, the dilemma regarding the mentioned issues- “Sartans, Nitrosamines, and Cancer”—became even more relevant and significant only in 2018, when the Food and Drug Administration (FDA) officially announced contamination of drugs for blood pressure, namely sartans, with nitrosamines, defined for decades as one of the most potent mutagens / carcinogens3.
We present a patient who received three different types of sartans in combination with hydrochlorothiazide for about 3 years, who subsequently developed lentigo maligna in the left cheek, as well as two dysplastic naevi. The potential role of systemic medication (sartans and thiazide diuretics) and eventual contaminants in these drugs (nitrosamines) and important pathogenetic inducers for the development of melanoma and pre-melanoma melanocytic skin lesions is discussed.
Case description
A 77-year-old Caucasian male, phototype II, visited our policlinic for Dermatology, Venereology and Dermatologic Surgery in February 2022 with a primary complaint of a pigmented lesion on the left cheek (Fig. 1A), first noticed in January 2020 and which increased in size and discoloration over the last 2 years. Arterial hypertension diagnosed 11 years prior has been under treatment with olmesartan/hydrochlorothiazide 20/12.5 mg id for 2 months in 2018, followed by irbesartan/hydrochlorothiazide 150/12.5 mg id for 3 months in 2019 and since then telmisartan/ hydrochlorothiazide 80/12.5 mg for about 2.5 years. No other drug history was reported.
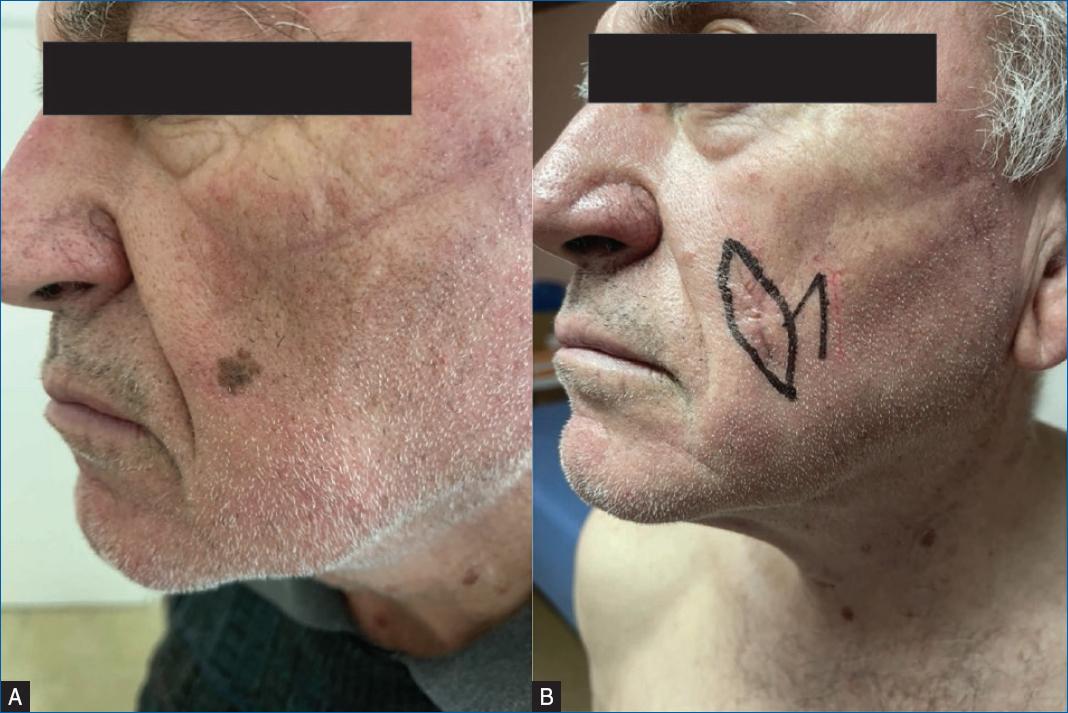
Figure 1 A: single superficially spreading, asymmetrical, hyperpigmented lesion on the left buccal region. Uneven borders, clear demarcation with varying shades of discoloration observed, confirmed as a melanoma on histopathology. B: clinical image of re-excision with preoperative surgical safety markings of an additional 1 cm in all directions.
Past medical history also revealed a prior malignant melanoma on the left arm surgically removed with an overall margin of 2 cm without performing an sentinel lymph node (SLN) biopsy with a thickness >2.5 mm with infiltration into the reticular dermis (Clark IV), without invasion of the lymphatic and venous vessels (T3aN0M0) and no evidence of disease progression on imaging studies on 2015, 2017, and 2019. Other compound nevi without dysplasia were removed on the left pectoral region back in 2015 and 2018. The patient reported no allergies or prior history of significant sun exposure, no family history of skin and other malignancies, including melanoma and social and living conditions were unremarkable.
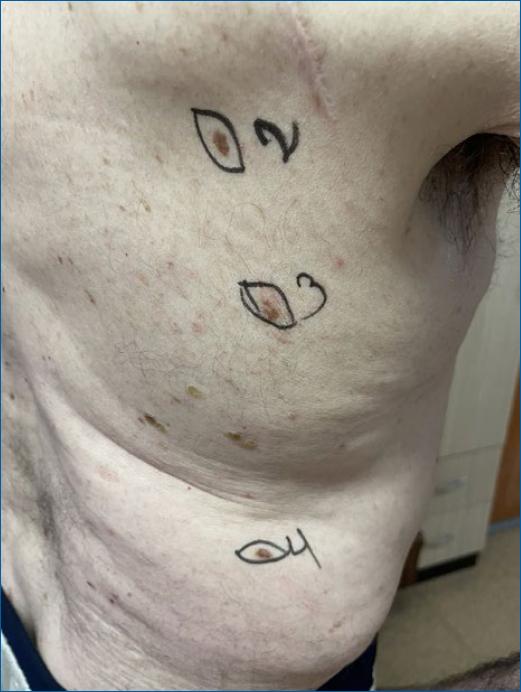
Dermatological examination in 2022 showed a superficially spreading solitary macule measuring roughly 0.9 cm in diameter on the patient’s left cheek region, next to the nasolabial fold (Fig. 1A) and three additional, irregularly shaped melanocytic naevi on the back (Fig. 2). The facial lesion was asymmetrical, unevenly bordered and with clear demarcated zones of varying shades of discoloration, mostly pronounced in its infero-lateral area, which according to the clinical and dermatoscopic evidence, was diagnosed as lentigo maligna. The other lesions localized on the scapular region, infra-scapular, and lumbar regions had positive ABCD criteria (asymmetry, irregular borders, discoloration, and diameter > 6 mm) (Fig. 2), and were diagnosed as melanocytic naevi with dysplasia.
With the suspicion that telmisartan/hydrochlorothiazide might contribute to the melanocytic lesions this medication was discontinued and an alternative treatment regimen of torsemide 10 mg/day, moxonidine 0.4 mg/day and flecainide acetate 100 mg 2id was prescribed with good blood pressure control.
The facial lesion was removed, under local anesthesia, in the form of an elliptical excision with a resection field of 0.1-0.3 cm and as histopathology confirmed a melanoma in situ, lentigo maligna type, a secondary excision for an additional surgical safety margin of 1 cm in all directions was performed (Fig. 1B) with clean resection margins. Histopathology of the three melanocytic naevi in the back confirmed dysplastic melanocytic naevi in two of them, with clean resection margins. Post-operative outcome was positive with good signs of wound healing for all four lesions. A 4-week follow-up visit showed no signs of complication or other concerns.
Discussion
As a subtype of melanoma in situ, lentigo maligna clinically presents in areas of sun-exposed skin and is more common in the elderly population4. This is due to chronic UV radiation as a key risk factor that has been shown to propagate BRAF, KIT, and TP53 mutations in melanoma4,5. Patients who develop melanoma are also at a higher risk of developing further or recurrent melanocytic lesions5. While many factors may influence the initiation and/or potentiation of such cancers, the exact etiological agent cannot be properly established5. As a result, identification of every possible risk factor is essential for achieving further cancer prevention. Antihypertensive drugs, such as sartans and thiazide diuretics, as well as non-antihypertensives such as ranitidine and metformin, have been found to be associated with an increased risk of developing both cutaneous as well as non-cutaneous cancers6,7,20,21. The exact mechanism is largely unknown but there is increasing evidence suggesting the contamination of these drugs with nitrosamine is the main culprit6,7.
One of the latest or most recent scientific publications from 2021 emphasizes the importance of nitrosamine- contaminated valsartan and the risk of cancer8. Statistical analysis indicates a 3-fold to 4-fold increased risk of developing cancer (per 100,000 people) in the presence of N-Nitrosodimethylamine (NDMA) or N-Nitrosodiethylamine (NDEA) contamination8. The first experimental data from 2018 linked sartans, and in particular losartan, with the possibility of potentiating metastasis of pre-existing melanoma cells in the laboratory9. The ability of sartans (again losartan) to potentiate melanogenesis/carcinogenesis was confirmed in a subsequent multi-center experimental study a year later10. The limitation of both laboratory tests is mainly the lack of data on whether the active substance used (losartan) contains or is contaminated with nitrosamines such as NDMA, NDEA for example.
Clinical data from retrospective observations available in the literature, are even more interesting and completely support this relation11,12. A retrospective analysis conducted in 2015 in patients taking sartans found that a long-term low dose of sartans was associated with a 53% risk of developing melanoma (OR: 1.53: 95% CI [1.05-2.23]), while long-term high-dose sartans have been associated with a 44% risk of developing melanoma (OR: 1.44: 95% CI [0.56-3.69])11. In 2017, an even more comprehensive retrospective analysis of an American team tracking the development of various forms of skin cancer after antihypertensive drugs concluded that:
Monotherapy with angiotensin receptor blocker could be associated with the development of melanoma, with a risk ranging between 24 and 225% depending on its stratification: unadjusted odds ratio (95% confidence interval): 2.25 (1.73-2.94)/adjusted OR (95% CI): 1.24 (0.54-2.85)12, and
Monotherapy with thiazide diuretic is associated with a relatively constant risk of developing melanoma (with and without risk stratification): unadjusted OR (95% CI): 2.06 (1.59-2.66)/adjusted OR (95% CI): 1.82 (1.01-3.82)12.
In both clinical studies, there were no data on whether patients were taking preparations contaminated with nitrosamines or “purely” free from this component11,12. Nevertheless, analyzing the shared data12, it could be concluded that in certain cases (combined use of sartans and hydrochlorothiazide) the risk of developing melanoma could be increased up to 4-fold.
Also, in the world literature described, there are several clinical reports of melanoma developing after monotherapy with sartans or sartans in combination with hydrochlorothiazide13-17. The single casuistry is also supported by large-scale, albeit still retrospective, follow-up of patients taking hydrochlorothiazide and developing melanoma, similar to the lentigo- maligna type, as in our case18. They found a 57% risk of developing lentigo maligna after taking hydrochlorothiazide18.
The potential contamination of diverse types of medications with nitrosamines has been associated since 2021 with a possible risk of developing melanoma in the bulletins of DRUG WATCH/FDA19,20. Therefore, in practice, it is quite possible that the nitrosamine NDMA, found in medications such as ranitidine or sartans (according to the FDA Bulletin 2021), can contribute to the potentiation/induction of melanoma19,20. Interestingly, however, and although there is no evidence in the literature (or no single publication) on the development of melanoma after taking NDMA-contaminated ranitidine, melanoma is listed in the 2021 FDA/Drug Watch bulletin as a potential candidate for compensatory claims related to the same drug- ranitidine19,20. However, for unknown reasons, NDMA does not appear in the FDA/DRUG WATCH compensation claims bulletin for medicines containing sartans or sartans/hydrochlorothiazide contaminated or potentially contaminated with NDMA. It is more conspicuous now, after Pfizer, one of the biggest pharmaceutical companies worldwide has conducted voluntary nationwide recalls of the drug quinapril hydrochlorothiazide due to the presence of nitrosamine above the acceptable daily intake (ADI) level21. With this data, alongside the vast recalls from the FDA, it is clearly evident that nitrosamine contamination within sartans, thiazide diuretics and other drugs like ranitidine and metformin is a major issue19,21.
It cannot be refuted that nitrosamines, as carcinogens, can initiate and even potentiate tumorigenesis in melanoma22. Studies have also shown that patients actively taking valsartan with a combination of hydrochlorothiazide have developed cancers including colon carcinoma, Kaposi sarcoma besides cutaneous melanoma23,24. While certain meta-analyses suggest “no-associated risk” between anti-hypertensives and skin cancer25,26, it is not clear whether the drug batches were contaminated with nitrosamines or not, which may also depend on the geographical regions and stricter controls of contamination during the manufacturing process. The vast diversity in such meta-analytical data, especially when there are other scientific publications, including meta-analyses, opposing these findings1,2,6-18, suggests perhaps an underlying issue is being missed. The step up in international inspections and the subsequent findings of elevated nitrosamine contamination, as well as the vast recalls of drug batches (by FDA, and now Pfizer) in antihypertensives and other commonly prescribed drugs such as ranitidine and metformin due to nitrosamine contamination merits further assessment by the scientific community19-21.
Although our patient did not report any prior history of sunburn, it is difficult to rule out the role UV exposure. But, it is important not to rule out other possible risk factors, especially the possible nitrosamine contaminated batches of olmesartan, irbesartan, and telmisartan in combination with hydrochlorothiazide (also a potential nitrosamine contaminated drug) that he took for 3 years.
With the recent introduction of permissible concentrations of nitrosamines in antihypertensive drugs, the additional risk of a cumulative effect also cannot be ruled out, especially as there have been three different drugs (in combination with hydrochlorothiazide) used by the patient27. Interestingly, foods, water, and tobacco that are known to have small amounts of nitrosamine have been unaccounted for and could tip the balance over the ADI level, even if trace amounts were found in each individual drug for example28. The lack of follow-up evidence especially with the calculation of such data can be problematic to discern the real culprit behind the pathological process of such cancers. Although these associations are difficult to ascertain from isolated case reports, it is important not to disregard the increasingly troubling data that ultimately affects the patient on an individual level and strengthen the relation between sartans, nitrosamines, and melanoma11-17.
In the present case, the patient developed a melanocytic lesion prior to starting his medication in 2019, therefore the pathological mechanisms are most likely multifactorial in nature, especially with his advanced age and possible long-term UV exposure. However, the possible role of sartans (in a combination of hydrochlorothiazide), particularly through nitrosamine contamination, should not be ignored, as nitrosamines are known not just to initiate tumorigenesis through induction of genetic mutagenesis but also to potentiate and facilitate neoplastic progression27-29. This potentiating effect could explain the short-term exposure in our patients. Additionally, photosensitization of the thiazide diuretic attributed to TP53 mutations and subsequent risk of melanoma may also play a role in the initiation of tumorigenesis alongside a cumulative effect UV radiation29. Such observations warrant a closer assessment of these drugs and addressing the nitrosamine contamination as well as the underlying synergism with other risk factors are the next steps in the right direction.
Conclusions
We present an interesting patient with arterial hypertension treated for approximately 3 years (2018-2022) with 3 different preparations containing olmesartan, irbesartan, and telmisartan, all in combination with hydrochlorothiazide, who developed lentigo maligna and dysplastic naevi. The potential role of sartans, nitrosamines, and hydrochlorothiazide as possible triggers of carcinogenesis are discussed.