Introduction
Sexually transmitted infections (STI) remain a major public health problem with an important burden worldwide. The World Health Organization (WHO) estimates that around 340 million people live with STIs worldwide every year. A health issue that recently led WHO to establish key strategic and operational transformations required to end STIs as public health concern by 20301.
Early in the 20th century, in response to the dramatic increase in the number of people with STIs against an economic crisis, genitourinary medicine emerged in Great Britain, aiming to provide healthcare to all facets of sexual health. Over time, people and their sexual behavior have changed, and so have STIs.2 Several factors influence the incidence, distribution, and types of STIs, including the consistently increasing travel abroad and population migration3,4. Foreign travel is in many ways related to the spread of diseases, and with the increasing affordability of air travel, there is a risk of the rapid globalization of emerging infections3. Immigration to Europe has also been identified as a health priority issue in Europe, as refugees, asylum seekers, and irregular migrants are particularly vulnerable to infectious diseases and may have worse health outcomes than the host population4.
Historical trends suggest that this phenomenon is not new and has had devastating consequences in certain populations. For instance, syphilis is believed to have been taken into Europe by explorers, and the globalization of human immunodeficiency virus (HIV) has also been helped by travel and migration5. Many reasons make foreign travel a risk factor for the acquisition of STIs. When abroad, people may feel less inhibited due to a perceived relaxation of social and moral constraints, leading to changing sexual behavior and exposure to STIs, with an estimated 20% of travelers having sex with new partners3. STIs acquired during international travel are more likely to be resistant to standard antimicrobial regimens, with the risk of higher sexual exposure and an increased chance that treatment regimens abroad may not be efficient in the local population, thus helping onward transmission of drug-resistant strains, such as Neisseria gonorrhea in Asia6.
In Europe, the increased migration flux heightens the chances of importing infections as migrants from different geographic areas may have higher incidence rates of STIs like hepatitis B and C and recombinant forms of HIV with drug-resistant profiles7. A total of > 30 million of Europe’s inhabitants have an immigration background, of which approximately one-third were born outside industrialized countries-regions with a different infectious diseases profile seen in Europe7.
The purpose of this review is to compile data from studies spanning Europe and dealing with some of the most impactful and rising imported STIs. The resulting overview of key STIs affecting migrant populations in Europe reflects the present state of knowledge in this field and may serve as a guide for planning public health policies and as an appeal for further research and prevention.
Syphilis
The bacterium responsible for syphilis is Treponema pallidum (T. pallidum) subsp. pallidum. Other names used for syphilis in the past include lues and the French disease, or hard chancre for primary syphilis. In recent years, syphilis has experienced a renaissance-like virtually no other STI8.
Two main hypotheses are proposed to describe the emergence of syphilis in Europe. The precolumbian hypothesis advocates that treponemal diseases have always had a global distribution9. In Europe, most of these conditions were mistaken for leprosy10. According to this theory, both syphilis and non-venereal treponemal diseases are variants of the same infections, and the clinical differences are the consequence of geographic and climate deviations and the degree of cultural and social development of populations within distinct areas. Briefly, pinta, yaws, endemic syphilis, and venereal syphilis are considered adaptative responses of Treponema to changes in the environment, cultural differences, and contact between various populations10,11. Yaws, endemic in Africa around 10,000 BC, would have remained unmodified in countries with similar climate conditions as those in the origin countries but would have developed into endemic syphilis in countries with colder and drier climates in which personal hygiene was overlooked and disregarded or into venereal syphilis in those areas where inhabitants exhibited a civilized society and paid more attention to personal hygiene9.
The Columbian hypothesis is a popular theory stating that the navigators in Columbus’s fleet would have brought the infection on their return from the New World around 1493 (Table 1)9,12. This theory is supported by documents belonging to Spanish physicians who were present at the moment when Christopher Columbus returned from America, who had already described the disease in some crew members on their return from the New World9. Further experiments have supported this hypothesis by finding evidence consisting of specific lesions on skeletal remains, such as luetic lesions, dated after Columbus’s journey in America13.
Table 1 First report dates of STIs in Europe
| STI | Date of the first report in Europe |
|---|---|
| Syphilis | 14939 * |
| HIV | 198516 |
| LGV | 198922 |
| Zika | 201329 |
| MPX | 202241 ** |
*According to the Columbian hypothesis.
**Date of sustained local transmission; there are sporadic reports of MPX infection in Europe previous to 2022. HIV: human immunodeficiency virus; LGV: lymphogranuloma venereum; STI: sexually transmitted infection.
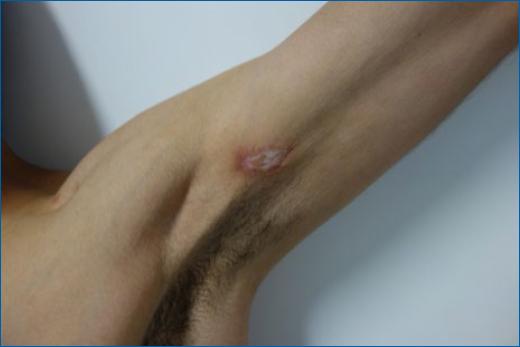
At the very beginning in Europe, syphilis was a disease of great severity and atypical evolution as compared to nowadays syphilis, with no rare fatal case9. The supporters of the Columbian hypothesis advocate that the severity of the condition was mainly due to its novelty, as the population had no time to gain immunity against the illness when venereal syphilis became endemic in Europe, certain strains of T. pallidum were selected, and the disease gained a milder course9. The hallmark of ancient syphilis probably is tabes dorsalis, the late neurological complications of syphilis, nowadays almost extinct14. Lues maligna, a rare form of ulceronodular secondary syphilis, is probably the presentation most similar to the first cases seen in Europe seen nowadays, mostly in immunocompromised patients (Figure 1)15.
Today, Europe is far from free from the syphilis epidemics, with the resurgence of syphilis over the last decades in high-income countries of the European Union and the European Economic Area (EU/EEA)8. For instance, the number of cases reported among men who have sex with men (MSM) in the EU/EEA has more than doubled (164% increase) from 2010 to 20168, thus supporting the need for public health programs that not only scout for emerging infections but also manage to know pathogens with an impactful burden.
HIV infection
In the 20th century, almost 500 years after the arrival of syphilis, the first cases of HIV in Europe were observed in 1985 (Table 1)16.
Human immunodeficiency virus (HIV) epidemiology in Europe is influenced by migration17. The epidemiology of migration-associated HIV reflects the disease in the migrants’ native countries. This relationship manifests in the transmission patterns and differences in the demographics and biometrics of the populations at risk16. European reports show that in 2015, for instance, 37% of all newly-diagnosed cases of HIV in the EU/EEA were in people born outside of the reporting country18. Low rates of testing and high rates of late diagnosis reflect gaps in HIV testing services for migrants as well as barriers to the provision and uptake of HIV testing services in this population18.
One of the most significant impacts of immigration on the HIV epidemic is the implication in the molecular epidemiology of the virus. Different features of HIV-1 molecular epidemiology, especially for the distribution of viral subtypes and for transmission of drug-resistant profiles, have been associated with immigration from north African countries17. Since their introduction, the subtype B clade has predominated in most Western and Central European countries, while the subtype A clade has been predominant in Eastern Europe19. HIV-1 subtype B has been responsible for what is often called the “Western epidemic” in Europe and has remained the dominant clade despite the introduction of non-B clades from later migrating populations19.
Migration from West Africa to Europe seems to be a potential source of HIV-1 non-B variant mobility, with a suspected route through the Maghreb and eventually reaching southern Europe, a region where the HIV-1 non-B variants have significantly increased in the past 10–15 years17. Drug resistance profiles are impacted by HIV genetic differences between different subtypes, reinstating the importance of continuous surveillance programs for the early detection of new variants spreading before they become more prevalent and permanently established17. Lastly, the need to identify circulating resistance profiles is essential not only for migrants but on the various infected populations to ensure a structured surveillance program.
Effective awareness-raising and prevention interventions for migrant populations most affected by HIV are essential to address this epidemic, as well as diminishing barriers to the provision and update of services for migrants.
Lymphogranuloma venereum (LGV)
Lymphogranuloma venereum (LGV) is an STI caused by L1, L2, and L3 serovars of Chlamydia trachomatis (CT) that classically manifest as an ulcer in the site of inoculation and lymphadenopathy; it can be transmitted through unprotected vaginal, anal, or oral sexual contact. LGV as a disease was described in 1833 to become a clinical entity only in 1913 by Durand, Nicolas, and Favre20. LGV is endemic in tropical and subtropical areas of the world (certain areas of Africa, Southeast Asia, India, the Caribe, and South America), with a reduced incidence in most developed countries20. Nonetheless, outbreaks have been reported in North America, Europe, and Australia, mainly as proctitis among MSM; and it is believed that LGV is substantially underdiagnosed in MSM across Europe21. One of the first published reports on LGV in Europe dates from 1989, when 27 cases of LGV were identified in Paris, the first in 1981 (Table 1)22. Of the 27 cases, 14 were natives from LGV-endemic countries. Since 2003 LGV has been reported endemic among MSM in some European countries23.
Early recognition and diagnosis are essential to ensure adequate and prompt treatment, which is currently longer in duration when compared to non-LGV CT, and to prevent LGV complications such as fissures, perirectal abscess, as well as systemic symptoms such as fever, fatigue, and weight loss21,23,24. Delayed LGV diagnosis is common in European countries due to several factors: availability of diagnostic tests is scarce, LGV is commonly misdiagnosed, and asymptomatic infection is not rare21. For instance, a recent report from three European countries that tested 500 specimens from CT-positive MSM rectal swabs found an LGV positivity of 25.6%21.
The lack of proper LGV diagnosis and surveillance hampers infection control measures, and it seems likely that LGV is continuing to be spread unchecked in MSM in many countries across Europe and beyond. Unified infection control efforts are needed to overcome barriers to implementing LGV testing, establish effective surveillance programs, and optimize diagnosis, treatment, and prevention of LGV.
Zika virus disease (ZIKV)
Zika virus (ZIKV) is an arthropod-borne virus from the Flaviviridae family. ZIKV was first isolated from a nonhuman primate in 1947, from mosquitoes in 1948 in Africa, and the first ZIKV infection in humans dated from 1954 in Nigeria25. Since then, ZIKV has spread across the globe, with the first reported outbreak of Zika fever in 2007 in the Federated States of Micronesia26. This was followed by other outbreaks in the Pacifica area, leading to 2015, when ZIKV caused an epidemic of unprecedented magnitude in the Americas, which resulted in recognition of the teratogenic effects of ZIKV on the developing fetal brain first reported in 2015 in Brazil27.
Most of the arboviruses, such as ZIKV, cause zoonoses that usually depend on nonhuman animal species for maintenance in nature, as humans are accidental hosts and an arthropod that acts as vector [mainly Aedes aegypti, and secondarily, Aedes albopictus (A. albopictus)]28. The most common mode of biological transmission is infection during a viremic blood meal and injection of infectious saliva during blood feeding (horizontal transmission)25. The capacity of arboviruses to adapt to new vectors may have a major impact on the geographic expansion of arboviruses28. Other non-vector-borne transmission modes include sexual transmission and maternal-fetal transmission25.
The first imported case of Zika fever in Europe was reported by a German traveler infected in Thailand in November 2013 (Table 1)29. In March 2016, surveillance of ZIKV disease started in EU/EEA27. The main objectives were early detection of locally-acquired cases in the EU/EEA and timely reporting of travel-associated cases, particularly those residing in areas of the EU/EEA where A. albopictus is established in order to trigger appropriate control measures27.
In 2019, France reported three autochthonous, vector-borne cases of ZIKV disease, thus establishing that A. albopictus in Europe is a competent vector of ZIKV30. Nonetheless, taking into consideration the changes in local populations and the limited window for transmission during the warmer months in the northern hemisphere, the real capability for sustained transmission remains limited27. However, climate changes will allow further expansion of the vector in Europe, especially in densely populated cities, through the heat island effect, a phenomenon that must be accounted for by ZIKV spread31.
The impact of ZIKV in Europe has been limited to returning travelers, a few sporadic locally-acquired cases due to sexual transmission, and for the first time in 2019, three autochthonous vector-borne transmissions27. Despite the evidence of limited competence of European A. albopictus populations in transmitting ZIKV, continued surveillance, with a particular focus on returning travelers, is mandatory to ensure early detection of risk areas and outbreaks, as well as an efficient public health response.
Mpox
In 1970, the Mpox virus (MPX-V), a zoonotic orthopox DNA virus related to the virus that causes smallpox, was first in the Democratic Republic of Congo32. MPX endemic transmission has been reported in some African countries, with few outbreaks and travel-associated cases outside Africa, always with limited secondary spread and human-to-human transmission33,34.
Since early May 2022, more than 52,000 MPX infections and 18 deaths have been reported in more than 102 countries, prompting the WHO to declare MPX an “evolving threat of moderate public health concern” on 23rd June 202235,36. The classic described mode of transmission of MPX-V is direct lesion-to-skin contact; nonetheless, there has been very little evidence of household spread of any form of MPX besides caregivers, which may indicate that this infection is not easily spread through casual contact and probably requires prolonged or repeated exposure, such as during sexual contact37. The recent 2022 outbreak is characterized by a papular skin eruption, fever, and lymphadenopathies (Figure 2), and most cases are mild and self-limited with no need for hospitalization or antiviral treatment; however, described MPX complications include pneumonitis, encephalitis, keratitis, secondary bacterial infections, deep tissue MPX abscess, myocarditis, and epiglottitis38–40.
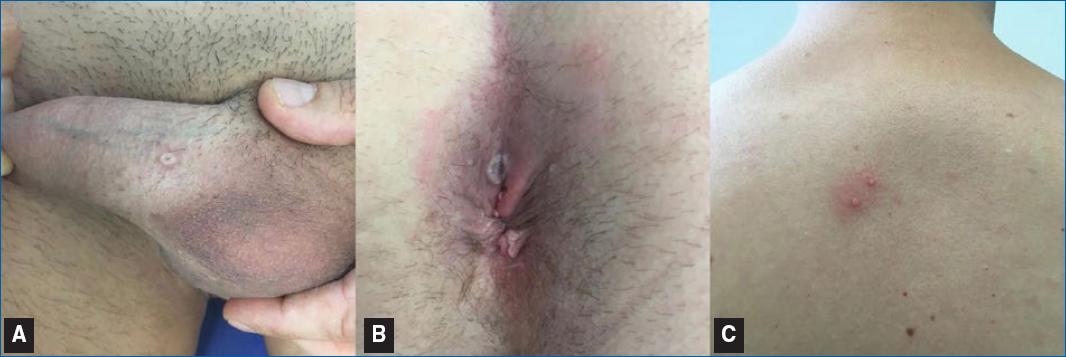
Figure 2 Clinical pictures of MPX infection. A: white umbilicated papule. B: whitish papule with a necrotic center in an erythematous background. C: two whitish papules in an erythematous background on the trunk.
The current MPX outbreak provides a new set of challenges to patients as well as to the healthcare and research communities. Previous lessons learned during the HIV and Covid-19 emergence should support the delivery of a more efficient and effective response to mpox. In turn, the response to MPX should strengthen the reaction to the inevitable next emerging or reemerging infectious disease of pandemic potential.
In Europe, MPX was first detected in the United Kingdom as an isolated case imported from Nigeria, an endemic country for MPX-V, followed by a hasty detection across other European Countries with an increasing number of cases (Table 1)41. Phylogenetic analyses suggest that the virus has circulated undetected for some time outside areas where it has been endemic, possibly masquerading as other STIs.42 The MPX 2022 outbreak also suggests changes in the biological characteristics of the virus, changes in human behavior, or both. These transformations are suspected to be driven by declining smallpox immunity, relaxation of coronavirus disease 2019 prevention measures, resumption of international travel, and sexual interactions associated with large gatherings40.
Conclusion
Over the last century, the world has experienced the impact that population movements can have on infectious diseases. As we move to an era where travel and migration are more accessible than ever before, we are expected to face new challenges when it comes to infectious diseases - and STIs are no different. The last few years have proved that the health authorities and providers must move on from the STI stigma and ensure timely infection management.
Improving access to healthcare for migrants arriving from highly endemic countries helps to identify, through screening, the groups most at risk for increased STIs prevalence while also being cost-effective in nature. Vaccination programs also provide a prevention strategy able to reduce disease burden. Integrating migrants into the local healthcare system ensures that disease cases are adequately managed while accurately defining incidence cases.
Because pathogens know no borders, the world needs to move cohesively and swiftly. Clinical care services must be expanded and strengthened, working in web-based systems to ensure that new pathogens are readily identified and targeted, safeguarding the population’s health.