Imiquimod, 3.75% cream, is a topical immunomodulator approved for treating actinic keratoses. Although local side effects are more frequent, it can also have systemic side effects. We report that imiquimod 3.75% can also produce erythema multiforme (EM), thus discarding it as an alternative to imiquimod 5% on this adverse effect.
An 81-year-old man, with a history of squamous cell carcinoma, presented with actinic damage and several actinic keratoses on the nose, cheeks, and frontal area. Treatment with imiquimod 3.75% was prescribed for all actinic keratoses to be applied once daily for two treatment cycles of 2 weeks, spaced by 2 weeks. In the 2nd week of the second cycle, the patient initiated an intense local reaction at the sites of application associated with erythematous lesions on the upper body, arms, and legs. Treatment was suspended. Physical examination revealed extensive areas of exudative and crusting lesions across the whole face (Figure 1A) and several round erythematous papules, in the central upper chest area, on the extensor surfaces of the forearms and lower legs, and the dorsum of the hands; some of these papules had central vesiculation or erosion, and others had a target morphology (Figure 1B). Mucosae, palms, or soles were not involved. The patient did not report a previous infection and was not taking any drugs other than his usual medication. Biopsy of a target lesion revealed abundant apoptotic keratinocytes, hydropic degeneration of the basal cell layer with band-like infiltration of leukocytes, and a slight perivascular lymphoid infiltrate in the papillary dermis, all consistent with EM (Figure 1C). Treatment with imiquimod was suspended. We initiated prednisone (30 mg) for 2 weeks and treated the face lesions with topical antibiotics. Treatment was effective, with complete clearance of the symptoms and cutaneous alterations within 3 weeks, and the diagnosis of EM induced by imiquimod was established.
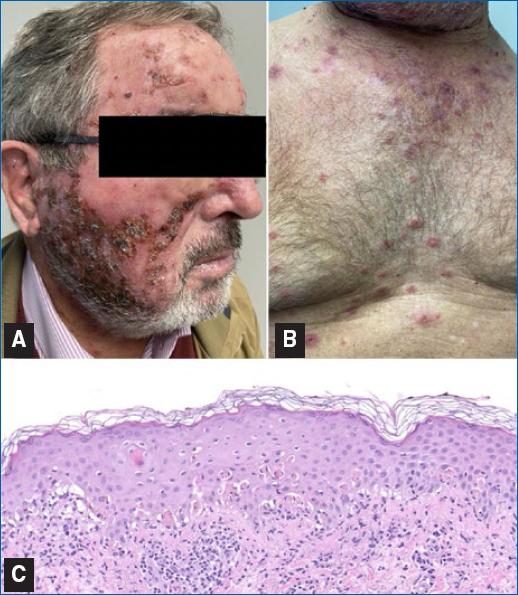
Figure 1 A: face, showing exudative and crusting lesions. B: anterior trunk, revealing target lesions. C: abundant apoptotic keratinocytes, hydropic degeneration of the basal cell layer with band-like infiltration of leukocytes, and a slight perivascular lymphoid infiltrate in the papillary dermis (HE 200x).
Imiquimod is a topical immunomodulator with antitumoral and antiviral activity approved for treating actinic keratoses. It stimulates innate and acquired immune responses by promoting the secretion of pro-inflammatory and antimicrobial cytokines leading to a T-helper 1 response and thus destroying tumors and virus-infected cells1. Although local side effects are more frequent, it can also have systemic side effects. We report that imiquimod 3.75% can also produce erythema multiforme, thus discarding it as an alternative to imiquimod 5% on this adverse effect.
The majority of imiquimod local side effects include erythema, crusting, and ulceration, frequently observed in the application area, which resolves satisfactorily on drug withdrawal. EM and other systemic eruptions were already described in patients under topical imiquimod. Many systemic drugs are associated with EM (nonsteroidal anti-inflammatory drugs, sulfonamides, anticonvulsants, and allopurinol), but few with topical treatments1–3. We found under 10 cases of EM associated with topical imiquimod, all with imiquimod 5%. This reaction is probably due to disproportionated systemic absorption and imiquimod immunomodulatory effects triggering a hypersensitivity reaction (type III or IV)2. An intense local inflammatory response to imiquimod, such as that experienced by our patient, was already proposed as a potential risk factor for systemic absorption, predisposing patients to EM3.
We conclude that despite its lower concentration, the 3.75% formulation can also induce EM-like eruptions, thus not being a valuable alternative to those who experience this reaction on the 5% formulation. This also supports an immunologically mediated mechanism of drug reaction. All previously reported cases with 5% imiquimod were in a heterogeneous group of patients, and it does not appear to be a consistent finding that could increase immunoreactivity (e.g., cancer). Genetic predisposition may be the driving factor, so studies on the human leukocyte antigens alleles could also be critical in finding predisposed patients.














