Introduction
Alopecia areata (AA) is a chronic autoimmune disease characterized by reversible, non-scarring hair loss. The most common pattern consists of hair loss in circumscribed, irregular patches of the scalp, which may progress to total scalp hair loss [alopecia totalis (AT)] or complete body hair loss [alopecia universalis (AU)]. Other less common forms of presentation include ophiasis AA, diffuse AA, and AA reticularis1,2.
Alopecia areata (AA) is the second most common cause of hair loss after androgenetic alopecia, affecting approximately 2% of the world’s population over the course of a lifetime. The prevalence is higher in children and adolescents, with both sexes being equally affected3. There is also a higher prevalence of AA in people with other autoimmune diseases such as vitiligo, atopic dermatitis, psoriasis, and thyroid disease4. The diagnosis of AA is clinical, but trichoscopy or, seldom, a skin biopsy may be necessary5.
Alopecia areata (AA) is a chronic and unpredictable disease in which patients experience periods of remission alternating with relapses. As a result, the quality of life is greatly affected, and AA is associated with a higher risk of psychological disorders such as depression and anxiety3.
Alopecia areata (AA) is considered a multifactorial disease involving complex interactions between genetic, environmental, and immunologic factors6. The pathophysiology of AA was largely unknown until recently, but several advances have been made in this area, particularly in relation to immune factors. Recent studies suggest that the loss of the immune privilege of the hair follicle is at the origin of AA. There is an activation of cluster of differentiayion (CD8+) T cells via the JAK-STAT pathway, and these cytotoxic T cells attack the hair follicle and disrupt its normal growth cycle1,2,6.
Currently, there is no curative treatment for AA. Traditional treatments are used off-label and have variable and transient efficacy, often with significant adverse effects4. According to international guidelines, the management of AA includes mostly topical, systemic and intralesional corticosteroids, and contact immunotherapy. The individual therapeutic approach depends mainly on age, severity, and stage of the disease (acute or chronic)5,7.
In most cases, topical and/or intralesional corticosteroids are the first line of treatment, especially in more localized forms of AA3,6,8. In refractory cases or in acute forms of severe AA, oral corticosteroids or, less frequently, intravenous corticosteroid pulse therapy may be used3,5. Prolonged use of these systemic corticosteroids is associated with multiple adverse effects, including weight gain, osteoporosis, and glucose intolerance, and therefore should only be used for short periods3,6. Contact immunotherapy with diphenylcyclopropenone or squaric acid dibutyl ester is mostly used as a second-line treatment in patients with chronic severe AA3,5,8. Additionally, topical anthralin is an alternative to corticosteroids in pediatric patients with severe AA3.
All these treatments are symptom-oriented and use their immunosuppressive and immunomodulatory properties unspecifically, which justifies their limited efficacy and safety3. However, the discovery of the importance of the JAK-STAT pathway in the pathophysiology of AA has led to the emergence of new, more targeted therapeutic options: JAK inhibitors. In recent years, several studies with JAK inhibitors have been conducted and have shown promising results in terms of efficacy and safety. Other innovative treatments, also targeting pathophysiological mechanisms, include apremilast, a phosphodiesterase-4 inhibitor, and biological therapies, such as dupilumab9.
Thus, given these recent advances, our aim is to review the scientific evidence regarding the pathophysiology of AA and data on the efficacy and safety of the new treatments for AA.
Pathophysiology
Disruption of the hair growth cycle
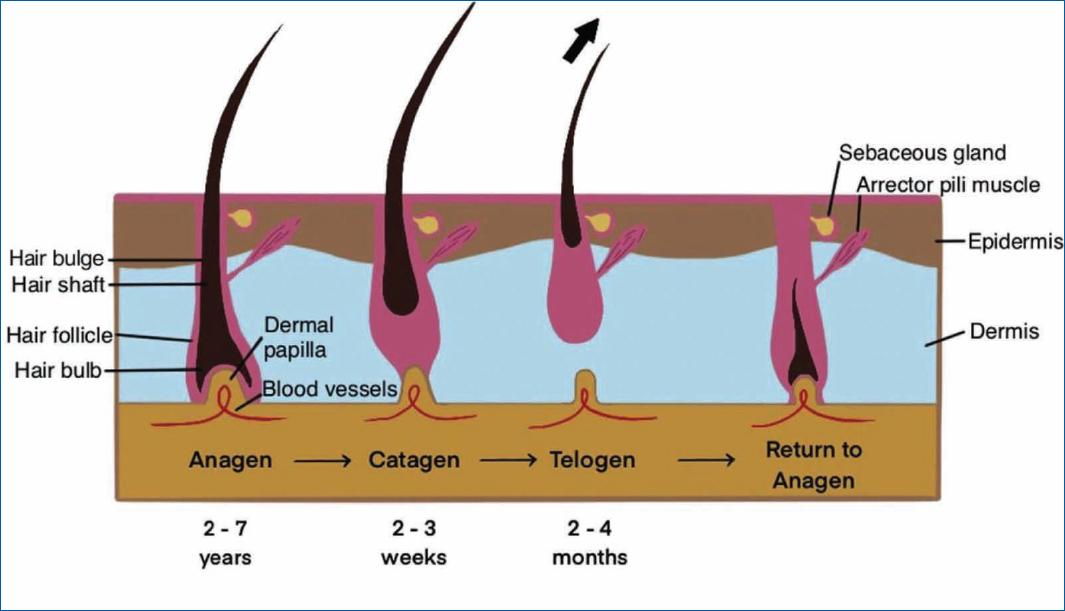
Alopecia areata (AA) is a disease that affects the hair follicle, a unique mini-organ that undergoes a cyclic and regenerative process throughout life3,10,11. The normal hair cycle is divided into three major phases-anagen (growth), catagen (involution), and telogen (rest)2,6,11-14. Some authors also consider a fourth phase, exogen, of hair shedding and return to anagen2,14.
Anagen is the longest phase of the hair cycle (lasting between 2 and 7 years) and is the phase in which most scalp hair (between 88 and 90%) is found at any given time in a person’s life. During this phase, the hair follicle actively receives nourishment from the blood vessels in the dermal papilla, leading to differentiation and proliferation of epithelial cells in the proximal direction, with hair growth. During catagen, the hair follicle begins to separate from the dermal papilla due to epithelial cell apoptosis. This is a transient process, lasting 2-3 weeks, and is followed by telogen, which lasts 2-4 months. At this phase, the hair follicle enters a period of relative quiescence and separates completely from the dermal papilla and, thereafter, from its only source of nutrients, culminating in hair loss (exogen phase). Then, there is a return to anagen, and a new hair cycle begins, which is repeated throughout life (Fig. 1)2,11,12. Keratinocyte and melanocyte stem cells, mainly located in the bulge area of the hair follicle, makes this cyclic process possible11.
However, in AA patients, there is a disruption in the normal hair growth cycle, with shortened anagen time and premature entry into the more advanced stages of catagen3,6,11,12,15 and telogen2,6,12-14, which is clinically manifested by hair loss.
Genetic susceptibility and environmental triggers
The exact cause of AA is unknown, but it is believed to have a multifactorial background, resulting from a combination of environmental influences and genetic factors involved in the immune system response10.
In fact, hundreds of single nucleotide polymorphisms have already been identified in AA patients, many of them in genes related to activation and proliferation of regulatory and CD8+ T cells, interleukin (IL) expression, and autoantigen presentation. Among them, it is important to highlight the human leukocyte antigen genomic region on chromosome 6, which encodes the major histocompatibility complex (MHC), as a major contributor to the AA phenotype16. Additionally, other genes located on the same chromosome, encoding the natural killer group 2D (NKG2D+) receptor and its danger ligands, have been shown to play an important role in the pathophysiology of AA, as discussed below13,16.
It is believed that in these genetically susceptible individuals, environmental triggers, such as physical or psychological stress, viral infections, or hair follicle microtrauma, can contribute to the dysregulation of immune mechanisms and the development of AA3. However, in most cases, no obvious trigger for the disease onset is found13.
Collapse of the immune privilege
In the hair follicle, there are immune-privileged areas, similar to other body regions such as the central nervous system, testicles, placenta, and eyes. The hair bulge during the entire hair cycle and the hair bulb only during the anagen phase is such immune-privileged areas, where antigens, although recognized by the immune system, do not generate an immune-inflammatory response17. This concept of immune privilege is essential for maintaining homeostasis and normal hair growth4.
Several mechanisms are responsible for maintaining immune privilege in the hair follicle–downregulation of MHC class I expression, which prevents the presentation of autoantigens to CD8+ T cells; production of potent immunosuppressants, such as transforming growth factor beta-1 (TGF β-1), IL-10, and insulin-like growth factor-1; increased expression of Fas ligand and programmed death ligand 1, pro-apoptotic molecules that target autoreactive T cells; and physical barriers, such as the absence of lymphatic drainage and the presence of a proteoglycan-rich, thick extracellular matrix that blocks the passage of immune cells4,6,17.
Dysregulation of these immune-tolerance mechanisms, caused by a local increase in IFN-γ, leads to the collapse of the hair follicle immune privilege, which is considered the major event in the pathophysiology of AA5,13,18,19. As a result, there is increased exposure of anagen hair follicle autoantigens to autoreactive CD8+ T cells that attack the hair follicle in the anagen phase, causing a premature transition to the catagen and telogen phases6,19. During the active phases of the disease, the immune attack is concentrated mainly in the bulb region, sparing the stem cells in the bulge area. This phenomenon explains the reversibility of hair loss in AA3,15.
More recently, Bertolini et al. have shown that not all AA is truly autoimmune and that a classic autoimmune cascade occurs only in a subset of AA patients19. In these cases, ectopic expression of melanogenesis-associated antigens recognized by CD8+ T cells leads to a local increase in IFN-γ signaling [“autoimmune, AA”]. In other patients, the pro-inflammatory activity of innate immune cells (NK cells, mast cells) induces IFN-γ production in a non-autoantigen-specific manner (“non-autoimmune”). Ultimately, both forms of AA coalesce in the collapse of hair follicle immune privilege, followed by increased antigen exposure and an influx of autoreactive immune cells. Thus, AA may represent a stereotypic response pattern to IFN-γ-induced hair follicle damage19,20.
In fact, there are profound changes in the hair follicle microenvironment in AA, with upregulation of danger ligands (activators of CD8+ T cells), presence of a robust immune infiltrate, increased levels of several pro-inflammatory cytokines, and increased expression of MHC class I and MHC class II10.
Overexpression of danger ligands, which activate a subpopulation of CD8+ T cells that express NKG2D+ receptors, is suggested as one of the mechanisms involved in the loss of immune privilege. These ligands include UL-16 binding protein-3 and MHC class I polypeptide-related sequence A. Recent studies have shown that hair follicles of AA patients have elevated levels of these two danger ligands compared to “healthy” hair follicles18,21.
In addition, there is a strong presence of an inflammatory infiltrate in the lesional hair follicles, composed mainly of CD8+ and CD4+ T cells, as well as Natural Killer (NK) cells, mast cells, and dendritic cells, which are described histologically as a “swarm of bees”. Among these immune cells, CD8+ NKG2D+ T cells stand out as the main effectors in AA and the first to infiltrate hair follicles3,19,21. These CD8+ NKG2D+ T cells have been shown to be necessary and sufficient to induce AA in murine models of the disease in a study conducted by Xing et al.37 Activated CD8+ T cells, in addition to their cytotoxic action through granzyme B, produce potent pro-inflammatory cytokines, such as IFN-γ and tumor necrosis factor-alpha (TNF-α), which prolong the immune-inflammatory state around and within the hair follicle10,16.
Local production of IFN-γ is considered the main mechanism responsible for the collapse of the hair follicle immune privilege5,19. This cytokine induces MHC class I and class II expression along the lower follicular epithelium, enhancing the presentation of autoantigens, respectively, to CD8+ and CD4+ T cells22. Moreover, IFN-γ induces the expression of CXC motif chemokines ligands 9 and 10, which attract and recruit circulating T cells, perpetuating the inflammatory state of the hair follicle17,22. There are other pro-inflammatory cytokines, such as IL-7 and IL-15, which are increased in AA and are essential for the survival of CD8+ NKG2D+ T cells and stimulation of their cytotoxic activity14,22.
Thus, AA is clearly associated with a type 1 immune response, with the involvement of IFN-γ as the main inflammatory mediator and activation of CD8+ and CD4+ T helper 1 (Th1) cells5,22,23. Th1 cells actively participate in the immune response by producing type 1 cytokines, such as IL-2, IL-12, IFN-γ, and TNF-γ23. Recent studies further suggest the involvement of Th2 (type 2 immune response) and Th17 cells in the pathophysiology of AA, as high levels of Th2 cytokines, such as IL-4 and IL-13, and Th17 cytokines, such as IL-17 and IL-21, have been detected in the serum of AA patients5,23. However, the exact role of these pro-inflammatory cytokines in AA is not so well understood, and more studies are needed to clarify it.
As in other autoimmune diseases, AA is associated with an impairment of cellular regulatory mechanisms, dysfunction and a decrease in the number of regulatory T cells, as demonstrated in a human study24.
Role of the JAK-STAT pathway
Many of these immunoinflammatory responses implicated in the pathophysiology of AA share a common intracellular signaling pathway–the JAK-STAT pathway3,4.
Janus kinase-signal transducer and activator of transcription (JAK-STAT) is an intracellular signaling pathway consisting of the receptor, Janus kinase (JAK), and signal transducer and activator of transcription (STAT). The JAK family consists of three JAKs (JAK 1-3) and a tyrosine kinase 2 (TYK2), while the STAT family contains seven STATs (STAT1-4, STAT5A, STAT5B, and STAT6). Depending on the ligand and receptor, different combinations of JAKs and STATs are activated3. When a specific ligand binds to the receptor in the cell membrane, the JAK protein phosphorylates its tyrosine component, activating its kinase function, which, in turn, phosphorylates the STAT component. This leads to the dimerization and activation of STAT, which translocates into the cell nucleus and acts as a regulator of the transcription of specific regions of DNA, modulating gene expression18.
In AA, the JAK-STAT pathway promotes the production of pro-inflammatory cytokines, such as IFN-γ and IL-15, with the participation of CD8+ NKG2D+ T cells and follicular epithelial cells. Indeed, CD8+ NKG2D+ T cells, activated by autoantigens, produce IFN-γ, which binds to its receptors on follicular epithelial cells and promotes the production of IL-15 through JAK-1 and JAK-2. In turn, IL-15, in combination with IL-15 receptor-α, binds to its receptors on CD8+ NKG2D+ T cells and leads to IFN-γ production via JAK-1 and JAK-3, completing the positive feedback loop that amplifies the local inflammatory response (Fig. 2)3,15,25.
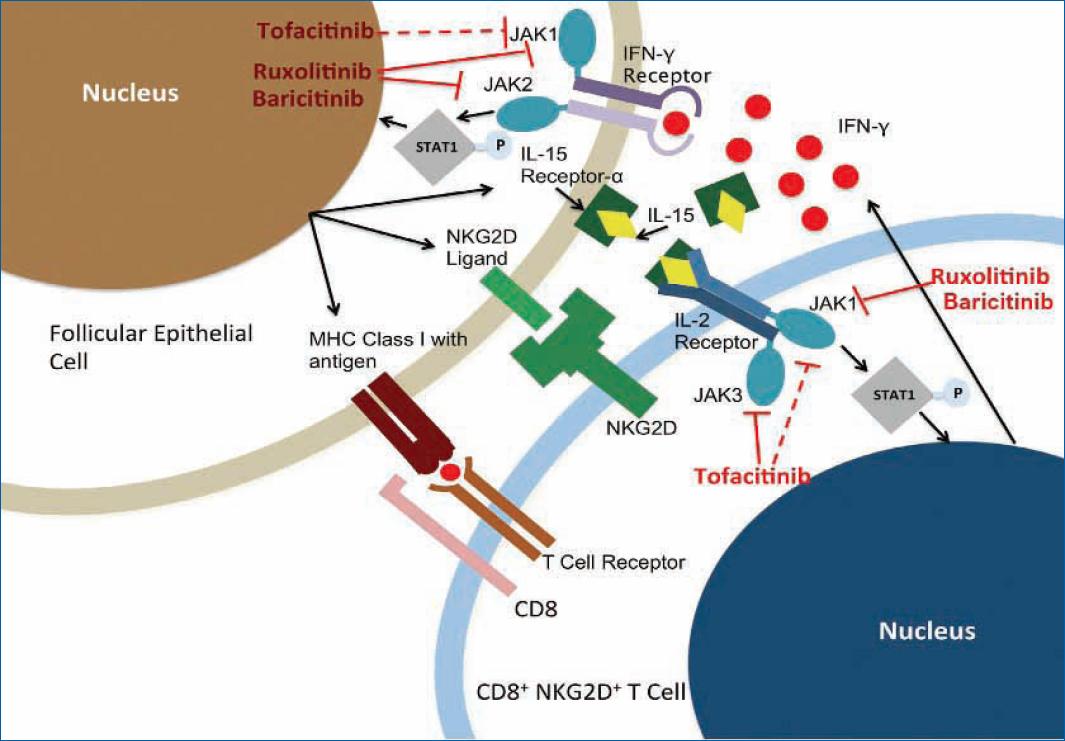
Figure 2 Interaction between CD8+ NKG2D+ T cells and follicular epithelial cells mediated by the JAK-STAT signaling pathway and the role of JAK inhibitors. Adapted from Strazzulla et al.25. Red bars represent the inhibitory capacity of JAK inhibitors. MHC: major histocompatibility complexes; NKG2D+: natural killer group 2D; IL: interleukin; JAK: Janus kinase; STAT: signal transducer and activator of transcription; IFN-γ: interferon-γ.
These findings have led to the development of drugs such as baricitinib, tofacitinib, and ruxolitinib (Fig. 2), which are JAK inhibitors and, therefore, potential treatments targeting AA, as discussed next25.
New treatments
Janus kinase (JAK) inhibitors
Janus kinase (JAK) inhibitors belong to a class of immunomodulatory drugs that have been widely studied over the years in the treatment of various inflammatory diseases, such as psoriasis, rheumatoid arthritis, and myelodysplastic diseases, and have recently attracted the attention of dermatologists for the treatment of AA6,9.
Janus kinase (JAK) inhibitors work by preventing the binding of adenosine triphosphate to the kinase domain of the JAK enzyme, an essential event for the phosphorylation of tyrosine residues. As a result, there is no tyrosine phosphorylation, and subsequent events in the JAK-STAT pathway are inhibited10. The researchers began to focus on JAK enzymes rather than other “players” involved in the pathophysiology of AA when it became clear that blocking cytokines such as IFN-γ or IL-15 was not sufficient to reverse AA, and the STATs could not be a good pharmacological target because they lacked catalytic activity26.
Janus kinase (JAK) inhibitors are divided into two groups–1st generation, the first to be developed, and 2nd generation, more recently developed. First-generation JAK inhibitors are less selective, showing activity against multiple JAK enzymes, while second-generation JAK inhibitors are more selective, inhibiting only one or two specific JAK enzymes6.
Before discussing JAK inhibitors in more detail, it is important to highlight a score created by Olsen et al. to standardize the assessment of AA severity in clinical trials–the severity of alopecia tool (SALT) score27. It is a simple and reproducible score that divides the scalp into four quadrants, each represented by a percentage (%) of its total area–left side (18%), right side (18%), top (40%), and back (24%). The percentage of hair loss in each quadrant is visually estimated and then summed to determine the SALT score–from 0 (no hair loss) to 100% (loss of all hair)27. A SALT score of 50% or greater has been defined as severe AA28. Most of the clinical trials we will review have hair growth as the primary outcome, using the post-treatment SALT score to make this assessment, or alternatively, the SALTn response, which compares the final SALT score to the baseline SALT score27.
Oral baricitinib
Baricitinib is a first-generation JAK inhibitor that preferentially inhibits JAK1 and JAK222. The efficacy of oral baricitinib in the treatment of AA was first suggested in 2015 in a case report of a patient with AA and chronic atypical neutrophilic dermatosis syndrome with lipodystrophy and elevated temperature who demonstrated complete hair regrowth after 9 months of treatment with baricitinib 11 mg/day29.
More recently, large-scale clinical trials have been conducted to evaluate the efficacy and safety of oral baricitinib in the treatment of AA. The first study (BRAVE-AA1), a phase 2, double-blind, placebo-controlled, randomized clinical trial (RCT), enrolled 110 patients with severe AA (SALT score of ≥ 50%) into three groups-placebo, 2 and 4 mg of baricitinib once daily (1id)30. The primary endpoint was the proportion of patients with a SALT score of ≤ 20% (considered a clinically successful treatment of severe AA). After 36 weeks of treatment, the proportion of patients with a SALT score of ≤ 20% was significantly higher in the baricitinib 2 (33.3%) and 4 mg (51.9%) groups than in the placebo group (3.6%) (Table 1). Adverse events observed were mostly mild30. Next, the two phase 3, double-blind, placebo-controlled RCTs (BRAVE-AA1 and BRAVE-AA2) evaluating the efficacy and safety of 2 and 4 mg doses of oral baricitinib included 654 and 546 patients, respectively, divided into three groups: placebo, 2 and 4 mg of baricitinib 1id31. A similar primary outcome assessed at 36 weeks (SALT score of ≤ 20%) showed that the proportion of patients with ≥ 80% hair regrowth in BRAVE-AA1 was 38.8, 22.8, and 6.2%, respectively, in the 4 and 2 mg, and placebo groups, while in BRAVE-AA2 it was 35.9, 19.4, and 3.3%, respectively (Table 1). These results were considered statistically significant (p < 0.001), and oral baricitinib was considered effective and also safe in the treatment of AA, with mostly mild adverse events at rates similar to placebo31.
Table 1 Summary of the main studies conducted with JAK inhibitors in the treatment of AA
| JAK inhibitor | Study type | Nº of patients | Dosing | Primary outcome | Response (%) |
|---|---|---|---|---|---|
| Oral baricitinib30,31 | Phase 2 RCT BRAVE-AA1 | 110 | Baricitinib 4 mg, 1id | SALT ≤ 20% week 36 | 51.9% |
| Baricitinib 2 mg, 1id | 33.3% | ||||
| Placebo, 1id | 3.6% | ||||
| Phase 3 RCT BRAVE-AA1 | 654 | Baricitinib 4 mg, 1id | SALT ≤ 20% week 36 | 38.8% | |
| Baricitinib 2 mg, 1id | 22.8% | ||||
| Placebo, 1id | 6.2% | ||||
| Phase 3 RCT BRAVE-AA2 | 546 | Baricitinib 4 mg, 1id | SALT ≤ 20% week 36 | 35.9% | |
| Baricitinib 2 mg, 1id | 19.4% | ||||
| Placebo, 1id | 3.3% | ||||
| Oral tofacitinib35 | Open-label clinical trial | 66 | Tofacitinib 5 mg, 2id | SALT50 3rd month | 32% |
| Oral ruxolitinib and tofacitinib38 | Open-label clinical trial | 75 | Ruxolitinib 20 mg, 2id | SALT50 6th month | 84.2% |
| Tofacitinib 5 mg, 2id | 78.4% | ||||
| Oral CTP-54339,40 | Phase 2 RCT | 149 | CTP-543 12 mg, 2id | SALT50 week 24 | 58% |
| CTP-543 8 mg, 2id | 47% | ||||
| CTP-543 4 mg, 2id | 21% | ||||
| Placebo, 2id | 9% | ||||
| Phase 3 RCT THRIVE-AA1 | 706 | CTP-543 12 mg, 2id | SALT ≤ 20% week 24 | 41.5% | |
| CTP-543 8 mg, 2id | 29.6% | ||||
| Placebo, 2id | 0.8% | ||||
| Phase 3 RCT THRIVE-AA2 | 517 | CTP-543 12 mg, 2id | SALT ≤ 20% week 24 | 38.3% | |
| CTP-543 8 mg, 2id | 33% | ||||
| Placebo, 2id | 0.8% | ||||
| Oral ritlecitinib and brepocitinib50 | Phase 2a RCT ALLEGRO | 142 | Ritlecitinib 200 mg/day, then 50 mg/day | SALT30 week 24 | 50% |
| Brepocitinib 60 mg/day, then 30 mg/day | 64% | ||||
| Placebo, 1id | 2% | ||||
| Oral ritlecitinib52,53 | Phase 2b/3 RCT ALLEGRO | 718 | Ritlecitinib 200 mg/day, then 50 mg/day | SALT ≤ 20% week 24 | 30.6% |
| Ritlecitinib 200 mg/day, then 30 mg/day | 22.3% | ||||
| Ritlecitinib 50 mg/day | 23.4% | ||||
| Ritlecitinib 30 mg/day | 14.3% | ||||
| Ritlecitinib 10 mg/day | 1.7% | ||||
| Placebo, 1id | 1.5% | ||||
| Topical ruxolitinib46 | Phase 2 RCT | 78 | Ruxolitinib 1.5%, 2id | SALT50 week 24 | 12.8% |
| Vehicle, 2id | 12.8% | ||||
| Topical delgocitinib47 | Phase 2 RCT | 31 | Delgocitinib 30 mg/gm, 2id | SALT50 week 12 | 11.8% |
| Vehicle, 2id | 16.7% |
RCT: randomized clinical trial; SALT: severity of alopecia tool; SALTn: ≥ n% improvement from baseline SALT score.
Positive results from the BRAVE-AA1 and BRAVE-AA2 studies led the Food and Drug Administration (FDA) to approve oral baricitinib (Olumiant) for the treatment of AA on 13th June 202232. As such, baricitinib is the first and only approved treatment for AA, marking an important milestone in the history of the disease, with a major impact on patients with severe AA10. Oral baricitinib is recommended for patients with severe AA at a dose of 2 mg/day, which may be increased to 4 mg/day if clinical response is inadequate. An initial dose of 4 mg/day may be considered in patients with AT/AU or with significant loss of eyelashes and/or eyebrows26. Before starting therapy, it is recommended to exclude the presence of tuberculosis and other active infections (hepatitis B and C and human immunodeficiency virus infection), and patients should be monitored for signs and symptoms of infection throughout treatment32. In addition, patients should undergo hematologic monitoring before and during treatment, including a complete blood count, coagulation studies, and a comprehensive metabolic panel. Moreover, they are contraindicated during pregnancy or breastfeeding, there is little safety data for young children, and lower dose limits are recommended for older individuals26.
Oral tofacitinib
Tofacitinib, the first to be developed, is a first-generation JAK inhibitor and the least selective, inhibiting all JAK enzymes (pan-JAK), although it has a preference for JAK1 and JAK36,10. The efficacy of oral tofacitinib was first suggested in 2014 in a case report of a patient with universal AA and concomitant plaque psoriasis treated with tofacitinib 15 mg/day, who experienced regrowth of all scalp hair at 3 months and all body hair at 8 months of treatment33.
Multiple studies have demonstrated the efficacy and safety of tofacitinib in AA, but most are low-quality studies such as case reports and case series, with a few retrospective studies and single-arm clinical trials10,26,34.
In 2016, an open-label, single-arm, clinical trial of tofacitinib 5 mg twice daily (2id) for 3 months in 66 patients with severe AA showed that 32% of patients were strong responders, with an improvement in baseline SALT score ≥ 50% (Table 1). Treatment was well tolerated, with limited adverse effects. However, 3 months after stopping tofacitinib therapy, all 20 responders had hair loss35.
More recently, a systematic review and meta-analysis showed that oral tofacitinib is an effective and well-tolerated drug in the treatment of AA. Longer treatment (> 6 months) was associated with a greater ≥ 50% SALT score improvement from baseline response (SALT50) in 62% of patients overall. Nevertheless, 3 months after tofacitinib discontinuation, most patients (74%) experienced a recurrence of AA, suggesting that the off-therapy clinical response is not durable36.
Oral ruxolitinib
Ruxolitinib is a first-generation JAK inhibitor that preferentially inhibits JAK1 and JAK2, similar to baricitinib26. The efficacy of oral ruxolitinib in the treatment of AA was first suggested in 2014 in a clinical trial of three patients with moderate to severe AA who showed almost complete hair regrowth after 3–5 months of ruxolitinib 20 mg 2id37.
Since then, several studies have been published demonstrating the efficacy and safety of oral ruxolitinib. In 2019, an open-label, comparative clinical trial was conducted with 75 patients with severe AA receiving either ruxolitinib 20 mg 2id or tofacitinib 5 mg 2id for 6 months. At the end of treatment, 84.2% of ruxolitinib patients and 78.4% of tofacitinib patients achieved a SALT50 response (Table 1). Both drugs were well tolerated. However, after 3 months of follow-up, about two-thirds of patients showed signs of relapse with hair loss38.
More recently, a deuterated form of ruxolitinib, deuruxolitinib (CTP-543), has been developed and evaluated in high-quality trials. The first study, a phase 2, double-blind, placebo-controlled RCT, involved 149 patients with severe AA divided into four groups—placebo, 4, 8, and 12 mg CTP-543 2id39. The proportion of patients achieving a SALT50 response after 24 weeks was defined as the primary outcome. At the end of the study, the proportion of patients with a SALT50 response was statistically significantly higher in the CTP-543 8 mg (47%) and 12 mg (58%) than in the 4 mg (21%) and placebo (9%) groups (p < 0.001) (Table 1). Adverse reactions observed were similar in all groups and were mostly mild to moderate39.
Therefore, the 8 and 12 mg doses of oral CTP-543 2id were selected to test its efficacy and safety in two phase 3 RCTs operated on a larger scale. The studies termed THRIVE-AA1 and THRIVE-AA2 enrolled 706 and 517 patients, respectively, who received CTP-543 12 mg, CTP-543 8 mg, or placebo 2id for 24 weeks40. The primary outcome in both studies (proportion of patients with a SALT score ≤ 20%) at 24 weeks of treatment showed ≥ 80% hair regrowth in THRIVE-AA1 in 41.5% in the 12 mg group, 29.6% in the 8 mg group, and 0.8% in the placebo group, whereas in THRIVE-AA2 it was 38.3, 33, and 0.8%, respectively (Table 1). In both studies, CTP-543 8 and 12 mg was statistically significant compared to placebo (p < 0.0001). Overall, CTP-543 was well tolerated and demonstrated a good safety profile40,41.
The positive results from these high-quality studies suggest that oral CTP-543 may be the next JAK inhibitor to be approved for the treatment of AA in the near future10.
Side effects of oral JAK inhibitors
As noted throughout this paper, oral JAK inhibitors appear to be safe and well-tolerated drugs for the treatment of AA. The most common adverse reactions are mild and include infections, such as upper respiratory infections, urinary infections, and folliculitis; diarrhea; acne; and headache42. Laboratory changes such as cytopenias, elevated low-density lipid and high-density lipid cholesterol, transaminases, and creatinine kinase have also been described but were overall uncommon and transient6,9,31,36. Herpes zoster infection is rare but has been observed in some studies31,36.
However, in the treatment of other inflammatory diseases, such as rheumatoid arthritis, where the safety profile of oral JAK inhibitors has been more extensively studied, their use has been associated with an increased risk of serious infection, thromboembolic and major adverse cardiovascular events, and cancer43,44. As a result of these findings, in September 2021, the FDA issued a “black box warning” for JAK inhibitors, the highest level of warning of all, to alert patients to the potentially serious risks associated with these drugs, mostly related to their potent immunosuppressive effects10,26,45. More recently, in October 2022, the European Medicines Agency’s safety committee (Pharmacovigilance Risk Assessment Committee) recommended further limitations on the use of JAK inhibitors in individuals aged 65 years or above, those at increased risk of cancer, major cardiovascular problems and deep vein thromboembolism, and current smokers or former long-term smokers46.
Still, to date, the various clinical trials conducted in AA suggest that oral JAK inhibitors appear to have a low risk of serious adverse events in this specific population, but long-term data is still not available. In addition, this may be explained by the fact that AA patients are generally younger and healthier, lower doses are used, and AA does not affect patients’ health as much as in other diseases where JAK inhibitors are used26,36. In any case, it is mandatory to warn patients about the potential risks associated with the use of oral JAK inhibitors and to monitor them throughout their treatment10,36.
Topical JAK inhibitors
Janus kinase (JAK) inhibitors have begun to be tested in topical formulations for the treatment of AA to limit systemic absorption and potential side effects of the oral route4,26. In 2020, a phase 2, double-blind, vehicle-controlled RCT evaluated the efficacy of topical ruxolitinib 1.5% 2id in 78 patients with moderate AA for 24 weeks. At the end of this period, only 5 of 39 patients in both the ruxolitinib and vehicle groups achieved a SALT50 response, leading to the conclusion that topical ruxolitinib has no significant effect in patients with AA (Table 1)47. Later, another phase 2 RCT demonstrated that topical delgocitinib (a pan-JAK inhibitor) 30 mg/gm 2id was not effective in the treatment of moderate to severe AA, as there was no statistically significant difference in SALT score reduction between the delgocitinib group and the vehicle group after 12 weeks of treatment (Table 1)48.
Recent meta-analyses and systematic reviews have also shown that topical JAK inhibitors are not effective in the treatment of AA42,49. In addition, topical JAK inhibitors appear to be even less effective than these reviews suggest, as approximately 50% of clinical trials of topical JAK inhibitors were terminated prematurely due to lack of efficacy or by sponsor decision, and their results were not published. Thus, these meta-analyses may have been subject to publication and selection bias, which could have been avoided by including the grey literature50.
Second-generation JAK inhibitors
Second-generation JAK inhibitors, which are more selective in their action, were developed with the goal of reducing the side effects of inhibiting multiple pathways34. There are four second-generation JAK inhibitors being studied for the treatment of AA–ritlecitinib, brepocitinib, upadacitinib, and abrocitinib10.
Oral ritlecitinib, an inhibitor of JAK3 and tyrosine kinase expressed in hepatocellular carcinoma, and oral brepocitinib, an inhibitor of JAK1 and TYK2, have been shown to be effective in the treatment of AA in a double-blind, placebo-controlled RCT named ALLEGRO. In the phase 2a study, 142 patients with severe AA were divided into three groups–ritlecitinib (200 mg/day for 4 weeks and 50 mg/day for the remaining 20 weeks), brepocitinib (60 mg/day for 4 weeks and 30 mg/day for the remaining 20 weeks) and placebo for 24 weeks51. After this treatment period, the proportion of patients achieving ≥ 30% SALT score improvement from baseline response (SALT30) was significantly higher in the ritlecitinib (50%) and brepocitinib (64%) groups than in the placebo group (2%) (Table 1). Overall, the drugs were well tolerated. However, two patients experienced a serious adverse reaction to brepocitinib51. In addition, after treatment discontinuation, patients experienced significant hair loss requiring therapy reintroduction within a medium of 16 weeks in the ritlecitinib group and 24 weeks in the brepocitinib group52. These positive results led to the initiation of the ALLEGRO phase 2b/3 study with ritlecitinib alone, which enrolled 718 patients with severe AA. At the end of 24 weeks of treatment, a statistically significant larger proportion of patients receiving the highest doses of ritlecitinib (30 or 50 mg/day, with or without a loading dose of 200 mg/day for 4 weeks) achieved a SALT score of≤ 20% compared to placebo (Table 1)53,54. The safety profile of ritlecitinib was consistent with previous studies. There is great interest in this drug because it is the only one that avoids JAK1 and JAK2 inhibition and their potential side effects (increased cholesterol and transaminases and cytopenias)55.
Upadacitinib and abrocitinib, both JAK-1 inhibitors used in atopic dermatitis, have less scientific evidence in the treatment of AA, with only a few case reports demonstrating efficacy10,26. Higher quality clinical trials will be needed in the future to understand the true role these 2 JAK inhibitors may play in the treatment of AA10.
Limitations of JAK Inhibitors
Although oral JAK inhibitors have proven to be an excellent therapeutic option in the treatment of AA, there are some limitations that must be considered.
First, as several studies have shown, after discontinuation of JAK inhibitors, AA rapidly relapses, and patients experience hair loss again35,38,51,56,57. This suggests that continuous treatment with these drugs is necessary to achieve a sustained off-therapy clinical response in AA6,8,22.
In addition, another concern with the use of JAK inhibitors in the treatment of AA is their high cost, which can reach $50,000/year per patient in the United States. This is much higher than any other treatment currently used for AA and may be unsustainable for the healthcare system, especially if JAK inhibitors have to be taken chronically6.
Phosphodiesterase-4 inhibitors (iPDE4)
Phosphodiesterase-4 inhibitors (iPDE4) are small molecules that prevent the hydrolysis and inactivation of cyclic adenosine monophosphate, thereby limiting the production of several pro-inflammatory cytokines58. Oral apremilast is an iPDE4 that has gained interest as a potential novel therapy in AA, as it has shown efficacy in some case reports59.
However, more recently, oral apremilast failed to demonstrate efficacy in the treatment of AA in a phase 2 RCT. In this study, 30 patients with severe AA were randomized to receive apremilast 30 mg or placebo 2id for 24 weeks. At the end of treatment, only one patient in each group achieved a SALT50 response, and there was no statistically significant difference in SALT score improvement between the apremilast and placebo groups (p = 0.38) (Table 2)57. These negative results corroborated the findings of a case series in which oral apremilast also demonstrated a lack of efficacy in the treatment of severe AA60.
Table 2 Summary of the main studies conducted with phosphodiesterase-4 inhibitors and biologics in the treatment of AA
| Drug | Study type | Nº of patients | Dosing | Primary outcome | Response (%) |
|---|---|---|---|---|---|
| Oral apremilast57 | Phase 2 RCT | 30 | Apremilast 30 mg, 2id | SALT50 week 24 | 8.3% |
| Placebo, 2id | 12.5% | ||||
| Subcutaneous dupilumab60 | Phase 2a RCT | 60 | Dupilumab 300 mg, weekly | SALT50 week 24 | 10% |
| Placebo, weekly | 0% | ||||
| Subcutaneous secukinumab63 | Phase 2 RCT | 11 | Secukinumab 300 mg weekly, then monthly | SALT50 week 24 | 0% |
| Placebo weekly, then monthly | 0% | ||||
| Subcutaneous aldesleukin64 | Phase 2 RCT | 43 | Aldesleukin at low doses, four cycles | SALT50 at 52 weeks follow-up | 14.3% |
| Placebo, four cycles | 9.1% |
RCT: randomized clinical trial; SALT: severity of alopecia tool; SALTn, ≥ n% improvement from baseline SALT score.
Biologics
In recent years, several biologics approved for the treatment of other inflammatory diseases have been tested as therapeutic options in AA, most notably dupilumab, secukinumab, and aldesleukin.
Dupilumab is a human monoclonal antibody approved for the treatment of moderate to severe atopic dermatitis that blocks the alpha subunit of the IL-4 receptor (IL-4R), thereby inhibiting two major Th2 cytokines, IL-4 and IL-1361. As mentioned above, the Th2 axis may play a pathogenic role in AA23,60. In addition, epidemiologic studies have shown a strong association between AA and atopic dermatitis, providing a therapeutic rationale for the use of dupilumab in AA62. However, the results of dupilumab in the treatment of AA are paradoxical. A recent systematic review showed that, on the one hand, 23 AA patients showed significant hair growth with dupilumab, but on the other hand, 21 were diagnosed with AA or had their preexisting AA worsened after treatment with dupilumab63. Amplification of the Th1 response resulting from inhibition of the Th2 axis has been hypothesized as the reason for this contradictory effect of dupilumab9.
More recently, a phase 2a RCT was conducted with 60 patients with moderate to severe AA who received weekly subcutaneous injections of 300 mg dupilumab or placebo60. At the end of 24 weeks of treatment, 10% of patients in the dupilumab group achieved a SALT49 response, compared with none in the placebo group (0%) (Table 2). In addition, patients with serum immunoglobulin E (IgE) levels ≥ 200 IU/ml and/or a personal or family history of atopy demonstrated a superior clinical response, suggesting that serum IgE measurement may be predictive of treatment response and aid in patient selection. The adverse effects observed were mostly mild61. Overall, the results of this clinical trial demonstrated the potential efficacy of dupilumab in the treatment of AA.
Secukinumab is a human IL-17A antagonist being investigated for the treatment of AA based on the potential pathogenic role of the Th17 axis in the pathophysiology of the disease23,64. In the only RCT conducted, 11 patients with severe AA were randomized to receive subcutaneous injections of 300 mg secukinumab or placebo, and none of the 11 patients achieved a SALT50 response at the end of treatment (Table 2). Based on these results, secukinumab was shown to be ineffective in the treatment of AA63.
Aldesleukin is a recombinant IL-2 that, at low doses, promotes the expansion and suppressive function of regulatory T cells, deficient in AA15,65. In a pilot study of 5 patients with severe refractory AA, partial hair regrowth was observed in 4 patients after administration of low-dose aldesleukin66. This led to an RCT of 43 patients with severe AA treated with the same dose of aldesleukin subcutaneously64. However, after 52 weeks of follow-up, no statistically significant difference was observed in the proportion of patients achieving the SALT50 score between the aldesleukin group and the placebo group (14.3 vs 9.1%, respectively) (Table 2). Thus, it can be concluded that low-dose aldesleukin does not appear to be an effective monotherapy for the treatment of severe AA64.
Conclusion
Alopecia areata (AA) is a chronic disease with an unpredictable nature that has a very negative impact on the quality of life of patients, with no curative treatment available yet. However, recent advances in understanding the pathophysiology of AA have led to a revolution in therapeutic options for AA. Available treatments have evolved from nonspecific immunosuppressive drugs with many systemic side effects to pathobiology-based drugs with improved specificity in their therapeutic targets and better safety profiles. At the forefront of these innovative treatments are JAK inhibitors, with oral baricitinib (Olumiant) being the first and only drug ever approved for the treatment of AA. In the coming years, more oral JAK inhibitors are expected to be approved for the treatment of AA, such as CTP-543 and ritlecitinib, which have shown efficacy and safety in a large, randomized phase 3 clinical trials.
Despite the fantastic opportunity in the treatment of AA that the advent of JAK inhibitors has brought, there are some concerns about their use that need to be addressed. JAK inhibitors are expensive drugs, which is set to remain one of the biggest barriers to their use in clinical practice.
In addition, patients do not show a sustained off-drug clinical response after discontinuation of JAK inhibitors, so long-term therapy seems to be necessary to maintain response. Given that this therapy may be administered indefinitely, further studies are needed in the future to assess the long-term safety of oral JAK inhibitors. Transitioning these patients to long-term regimens with second-generation oral JAK inhibitors or topical JAK inhibitors is an alternative that should be explored, as they appear to have less potential for systemic side effects. However, topical JAK inhibitors studied until now have shown to be ineffective in the treatment of AA. Therefore, it might be important to find ways to improve the skin penetration of these drugs so that they can still play a role in the treatment of AA. Oral second-generation generation JAK inhibitors, on the other hand, have demonstrated efficacy in clinical trials and are expected to be added to the AA therapeutic armamentarium soon.
Other innovative treatments, such as phosphodiesterase-4 inhibitors and biologics, appear to have more limited efficacy in the treatment of AA. Among these, dupilumab stands out as being more effective than the others, especially in patients with atopic features. However, further studies are needed to clarify its potential paroxysmal effects on AA.
In the coming years, as more of the pathophysiological mechanisms of AA are unveiled, the number of therapeutic options available to treat this very challenging disease is expected to grow significantly.