Introduction
Despite its rising incidence in most European countries over the last decade, penile cancer is still considered a rare disease1. It is frequently underrecognized by clinicians leading to delays in diagnosis and treatment2,3. Additional reasons for delaying diagnosis are attributed to patient factors, as usually, patients defer seeking medical advice due to mild symptoms, feelings of embarrassment, guilt, fear, denial, and lack of awareness1,4. It is estimated that 15 to 60% of patients postpone clinical observation for at least 1 year after the first signs of the disease2. This underlines the importance of consciousness regarding the condition, particularly for dermatologists and urologists most sought by the patient for these lesions. Prompt diagnosis is key for appropriate and early treatment, reducing the morbidity and mortality from penile cancer.
Squamous cell carcinoma (SCC) is the predominant pathological entity, representing 95% of all penile cancers. The estimated 5-year overall survival is 66%5. Delay in diagnosis impacts prognosis1.
Epidemiology
Penile SCC had a global estimated burden of 36,068 cases in 20203. Incidence rates have marked geographical variability2,5,6. Whereas the prevalence in developed countries is less than < 1/100,000, it can reach 10% of all cancers in men in low and middle-income regions, explained by many social, hygienic, and cultural factors3,4,7. In developed countries, namely in Europe, a rise in penile cancer incidence has been reported7. Penile SCC usually occurs in men aged between 50 and 70 years1,8. Nevertheless, it can also occur in younger patients, especially if associated with HPV1,7.
Risk factors
The most important risk factor for penile cancer is HPV infection, especially by oncogenic subtypes, such as HPV 16 or 18 and eventually 31, 33, 45, 56, and 659. HPV prevalence is estimated at around 50%9, and the relative risk for penile cancer is approximately 4.5 higher in HPV-seropositive patients10. However, the impact of HPV infection on penile cancer diagnosis, prognosis and prevention still warrants further research.
Although genital warts are generally associated with infection with low-risk HPV types, premalignant and malignant lesions have been found within genital warts11,12. Genital warts can constitute risk markers for the development of other HPV infections, as they indicate high-risk sexual behaviors10. Thus, close follow-up of patients with sexually transmitted infections (STIs), namely anogenital warts, should be considered to assess the risk of developing malignant lesions.
Besides a history of STIs, other major risk factors for penile cancer include phimosis, chronic inflammatory dermatoses such as lichen sclerosus, poor genital hygiene, ultraviolet A phototherapy, obesity, smoking, immunosuppression, low socioeconomic status, and low educational level13,14.
Basically, two major pathogenic pathways have been proposed, one linked to HPV and another linked to chronic inflammation1,6. Based on this, the 2022 World Health Organization classification recommends the subdivision of penile SCC into HPV-dependent and HPV-independent types15. This classification recognizes an association between histological variants and HPV: basaloid, papillary-basaloid, warty, warty-basaloid, clear cell and lymphoepithelioma-like carcinomas are considered HPV related; common type, carcinoma cuniculatum, verrucous, papillary, pseudohyperplastic, pdeudoglandular, adenosquamous and sarcomatoid carcinomas are considered HPV-independent5,15,16. However, diagnosis based solely on morphological criteria may be misleading in a small proportion of tumors, and HPV deoxyribonucleic acid (DNA) testing and/or p16 immunostaining is required to classify SCC as HPV-associated or HPV-independent5 properly. The expression of p16 is correlated with the integration of HPV’s viral genome into the intracellular host genome17. Therefore, currently, p16 expression found in penile intraepithelial neoplasia (PeIN) or invasive SCC is considered a surrogate marker for high-risk HPV infection17,18. Additionally, the role of p16 as a prognostic marker is currently under investigation, as some works have shown that men with HPV or p16-positive penile cancer have a survival advantage17,18.
Precursor lesions and HPV
Penile intraepithelial neoplasia (PeIN), a precursor lesion for penile cancer, is usually classified according to the degree of dysplasia, namely PeIN I if mild dysplasia is present, PeIN II in moderate dysplasia and PeIN III when dysplasia is severe or carcinoma in situ19. Like SCC, PeIN can also be classified as HPV-related or non-related15. Although these lesions are clinically similar, the association with HPV can be relevant as it could potentially guide treatment and enhance follow-up strategies. The pooled HPV DNA prevalence in PeIN was 79.8%19, higher than its prevalence in invasive SCC, suggesting that HPV infection may be associated with a less aggressive evolution and with a more predictable carcinogenic path20.
Clinical aspects
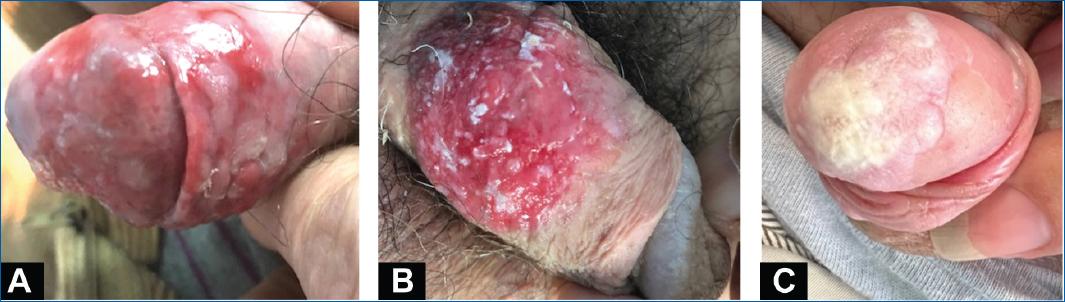
Around > 50% of penile SCC arises in the glans, followed by the prepuce, both glans and prepuce, coronal sulcus and the shaft2. Clinical presentation can vary, but SCC usually manifests as an erythematous area of induration or an ulcerating infiltrative lesion (Fig. 1)16. In premalignant lesions, changes can be more subtle, such as an erythematous patch with variable degrees of infiltration (Fig. 2)16. Bowenoid papulosis, Bowen’s disease and erythroplasia of Queyrat are three clinically recognized manifestations of carcinoma in situ21. The former is characterized by multiple red-brown papules, sometimes coalescing into a plaque. Bowen’s disease presents as a pink plate with white scales, and erythroplasia of Queyrat manifests as an eroded erythematous plaque with well-demarcated borders usually arising in the glans or prepuce21. Early suspicion and biopsy are necessary to prevent delays in diagnosis and treatment1. This is particularly relevant in patients with chronic genital dermatosis, like lichen sclerosus. Faced with a persistent suspicious lesion, particularly one with little or no response to corticosteroids, one should have a low threshold to perform a biopsy16.
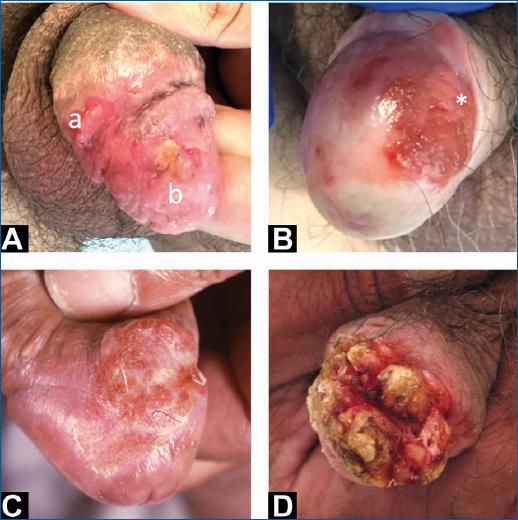
Figure 1 A: erythematous infiltrated plaque corresponding to PeIN 2 (a) and invasive SCC (b); B: erythroplasia of Queyrat (SCC in situ) with an area (*) of invasive SCC; C: area of induration in a patient with lichen sclerosus, corresponding to invasive SCC; D: invasive SCC manifested by a verrucous exophytic lesion distorting normal anatomy of glans and prepuce. PeIN2: penile intraepithelial neoplasia grade 2; SCC: squamous cell carcinoma.
Diagnosis and staging
Confirmation of the diagnosis by biopsy of the suspected lesion and histopathological examination should be followed by staging1. Penile SCC should preferably be staged according to the American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) eighth edition tumor, nodes, and metastases classification (Table 1).
Table 1 AJCC/UICC 8th edition for clinical and pathological staging
| Primary tumor (T) | |
|---|---|
| T-category | T criteria |
| Tx | Primary tumors cannot be assessed |
| T0 | No evidence of a primary tumor |
| Tis | Carcinoma in situ (PeIN) |
| Ta | Noninvasive localized SCC |
| T1 | Glans: tumour invades lamina propria Foreskin: Tumor invades dermis, lamina própria or dartos fáscia Shaft: Tumor invades connective tissue between epidermis and corpora |
| T1a | Without lymphovascular or perineural invasion and is not high grade (G3 or sarcomatoid) |
| T1b | With lymphovascular and/or perineural invasion or is high grade (G3 or sarcomatoid) |
| T2 | Tumour invades corpus spongiosum with or without urethral invasion |
| T3 | Tumour invades corpus cavernosum with or without urethral invasion |
| T4 | Tumour invades adjacent structures (scrotum, prostate, pubic bone) |
| Regional nodes (N) | |
| Clinical N category | Clinical N criteria |
| cNx | Regional LNs cannot be assessed |
| cN0 | No palpable or visibly enlarged inguinal LNs |
| cN1 | Palpable mobile unilateral inguinal LN |
| cN2 | Palpable mobile ≥ 2 unilateral inguinal LN or bilateral |
| cN3 | Palpable fixed inguinal nodal mass or pelvic lymphadenopathy unilateral or bilateral |
| Pathological N | Pathological N criteria |
| pNx | LN metastasis cannot be established |
| pN0 | No LN metastasis |
| pN1 | ≤ 2 unilateral inguinal metastases, no extranodal extension |
| pN2 | ≥ 3 unilateral inguinal metastases or bilateral metastases, no extranodal extension |
| pN3 | Extranodal extension of LN metastases or pelvic LN metastases |
| Distant metastasis (M) | |
| M category | M criteria |
| M0 | No distant metastasis |
| M1 | Distant metastasis present |
| Histopathological grading (G) | |
| Gx | The grade of differentiation cannot be assessed |
| G1 | Well-differentiated |
| G2 | Moderately differentiated |
| G3 | Poorly differentiated/high grade |
AJCC/UICC: American Joint Committee on Cancer/Union for International Cancer Control; T: primary tumor; SCC: squamous cell carcinoma; N: regional nodes; M: distant metastasis; G: histopathological grade of differentiation.
Physical examination should include inguinal LN palpation16. In obese patients, the limitations of clinical evaluation can be overcome through ultrasound examination of inguinal LN6. When enlarged, LN is detected on physical examination; LN metastases can be diagnosed by percutaneous fine-needle aspiration cytology4.
In clinically unremarkable inguinal LNs, management is particularly challenging because, in up to 25% of cases, inguinal lymphatic micrometastases are present1,6. In these cases, a dynamic sentinel node biopsy (DSNB) is recommended in intermediate (T1G2) or high-risk (T1G3 or worse) disease4. The sensitivity of DSNB is approximately 90-95% for micrometastases detection, with low associated morbidity6. In centers where DSNB is not available, modified inguinal lymphadenectomy is a safe and appropriate alternative6,22.
When positive LN is detected, staging for systemic metastases is recommended through computed tomography of the thorax, abdomen, and pelvis16. A positron emission tomography scan is an acceptable alternative with high sensitivity and specificity in the detection of distant metastases; however, limited spatial resolution reduces its acuity for small metastases. Additionally, false positives may occur due to inflammation23.
Treatment
Given the lack of randomized controlled trials, multidisciplinary care in experienced centers is crucial for improving outcomes16.
Previously, the mainstay of treatment in localized disease was excision with wide margins (2 cm)1. However, current recommendations allow narrow tumor margins as long as complete excision is achieved1. For carcinoma in situ, topical immunotherapy or chemotherapy (imiquimod applied once daily or on alternate days, 5-fluorouracil applied on alternate days for 6 weeks24), as well as epithelial ablative techniques (cryosurgery, CO2 laser, neodymium-doped yttrium aluminum garnet laser or photodynamic therapy) are treatment options1. For low and intermediate-grade T1 lesions, circumcision, wide local excision or partial glansectomy are recommended16. However, high-grade T1 or T2-T3 disease requires more extensive surgical interventions, with partial or total penectomy1,16. Mohs surgery could play a role in smaller lower-grade tumors, achieving a superior esthetic and functional result1,25. Its use in larger, stage II or above tumors should be discouraged since these cases are not suitable for penile-sparing therapy25.
Squamous cell carcinomas (SCCs) are generally radiosensitive tumors1. Thus, radiotherapy, particularly brachytherapy, can be considered an organ-sparing alternative16. This modality is reserved as the initial treatment for invasive T1 and T2 cancers. Despite local recurrence rates ranging to 20% after 5–10 years, secondary control could be achieved by salvage surgery in 85% of cases4. Radiotherapy is also advocated as an adjuvant treatment to the inguinal lymphatic area when histopathological examination reveals more than one metastatic LN or extranodal extension4.
The role of chemotherapy in penile SCC remains under discussion, as most available evidence comes from small prospective or retrospective studies4. Further high-quality prospective studies are required. Cisplatin has been the cornerstone of the combination regimens used4. Neoadjuvant chemotherapy (NC) is recommended in patients with fixed or bulky inguinal LN, bilateral LN involvement, or pelvic node involvement16. Similarly, adjuvant chemotherapy is advocated for patients that had not received NC in pN2-pN3 disease4,16.
Palliative therapy is the standard of care in patients with unresectable locally advanced or metastatic disease16. Studies show that palliative chemotherapy can achieve limited survival benefits1.
Prognosis
The overall 5-year survival rates are above 90% for pT1 tumors, decreasing to 55% for pT3 and under 50% for patients with positive LN pN1-N31. Patients with metastatic disease have a poor prognosis, with a median overall survival of 7–8 months16. Around > 90% of recurrences occur in the first 5 years, so patients should be carefully followed in this period, with follow-up visits every 3 months in the first 2 years and every 6 months in the remaining 3 years4. The recommended follow-up depends on nodal involvement, varying from physical examination alone to regular imaging, such as CT, MRI or ultrasound with fine needle cytology1.
Future perspectives
The protective effect of HPV vaccination against cervical cancer is reported in various studies; however, in penile cancer, its impact is inconsistently described26–28. Vaccination in males is recommended by several international scientific societies and is now being implemented in many countries, including Portugal29,30. The impact of this preventive measure is promising and expected to be clarified in the next few years31.
To improve early diagnosis, identify therapeutic targets and support prognosis evaluation; recent research has identified several tissue and serum biomarkers. Nevertheless, significant gaps still exist in understanding the potential clinical implications of each biomarker8.
Several novel therapies are under investigation for the treatment of advanced-stage disease16. Phase II studies, including targeted therapies (e.g., EGFR inhibitors and immune checkpoint inhibitors), are ongoing with promising preliminary results16. A basic understanding of penile SCC at a molecular level holds promise in developing novel therapeutic approaches16.
Conclusion
Given its rarity and low levels of awareness by both patients and clinicians, penile SCC represents a diagnostic challenge. Prompt SCC diagnosis is critical for effective treatment since prognosis in the early stages is excellent. Furthermore, clarification of the role of HPV in premalignant lesions and penile SCC pathology has the potential to improve prevention and treatment regimens.