Introduction
The diseases SJS and TEN are rare but life-threatening mucocutaneous conditions considered a continuum of the same disease1, characterized by keratinocytes necrolysis, and that are distinguished by the proportion of detached body surface area–SJS involves < 10% of the total body surface area and TEN involves > 30%; between 10 and 30% it is designated as SJS/TEN overlap2. These conditions are drug-induced in about 60-90% of cases2-4. Children aged 11-15 years and polymedicated patients2 have the highest incidence. ASD are known to cause SJS1,5, but only a very few ethosuximide-induced SJS cases have been reported5-7.
Clinical case
A 4-year-old boy was brought to a level 2 pediatric emergency service with a 2-day history of high fever associated with prostration and anorexia. A total of 18 days before, he was admitted to the pediatric ward due to 7 days of fever associated with a generalized rash (that spared palms and soles), with discharge after clinical improvement with apyrexia.
From personal history to highlight-refractory epilepsy with myoclonic absences, encephalopathy, global development delay, poor stature-weight progression, microcephaly, hypotonia, and ostium secundum atrial septal defect, surgically corrected without a residual shunt. Followed in pediatric neurology consultations at age 2, he was medicated with sodium valproate (40 mg/kg/day), clonazepam (0.05 mg/kg/day), and levetiracetam (40 mg/kg/day); he had started ethosuximide (40 mg/kg/day) 9 days before the onset of the symptoms due to epileptic seizures refractory to ASD. No recent history of immunization was found.
On physical examination, he presented a macular rash (mostly on the face, trunk, limbs, palms, and soles). Laboratory findings showed a white blood cell count of 7.10 x 109/L (55.9% segmented neutrophils and 3.1% eosinophils) and a C-reactive protein of 3.94 mg/dL. Reverse transcriptase polymerase chain reaction for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and hemoculture were negative. Serologies for Epstein-Barr virus and Cytomegalovirus were compatible with immune status.
He was admitted for surveillance, maintaining a high fever and rash worsening associated with prostration. On the 3rd day of hospitalization (5th day of high fever), he presented with coalescing maculopapular rash that merged on patches and plaques and some atypical target lesions (Figures 1 A-C). Bullous lesions on the flexures, mostly on knees (Fig. 1 D), with positive Nikolsky sign, lip erosions, and edema, with crusted and bleeding lesions (Figures 1 E and F); bilateral eyelid edema (Figures 1 E and F) and oliguria.
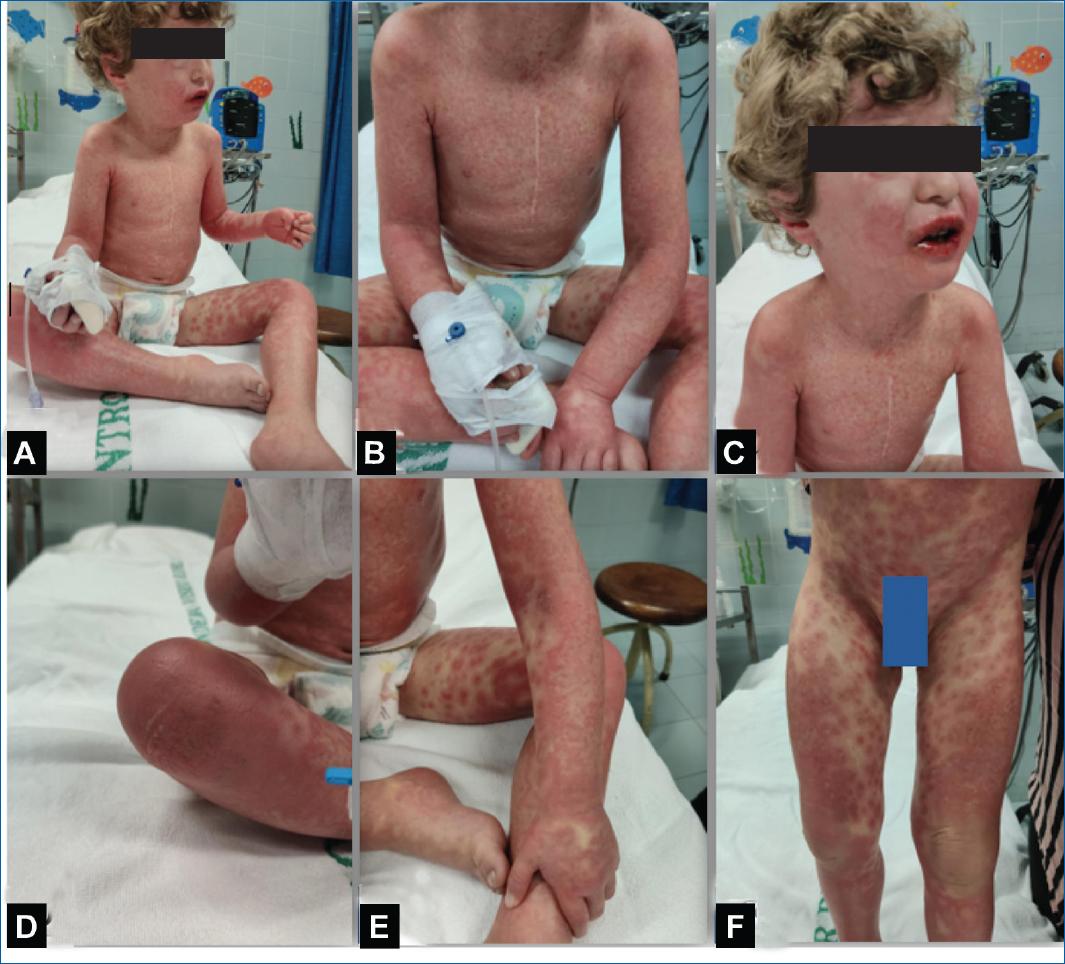
Figure 1 A, B and C: clinical presentation on the 5th day of fever with coalescing maculopapular rash, very confluent with target lesions, and D: bullous lesions in the flexures, mostly on knees and with E, F: bilateral eyelid edema and lip edema with crusted and bleeding lesions.
The case was discussed with the dermatology and neuropediatrics teams, and due to the suspicion of serious adverse skin reaction associated with ethosuximide, this therapy was discontinued, and he started IVIG (2 g/kg).
Due to the worsening of the general condition with 15% of detached body surface area, he was transferred to a level 3 hospital and initially admitted to the Pediatric Intensive Care Unit (PICU). He remained apyretic and hemodynamically stable. Fulfilled 7 days of parenteral nutrition, with progressive tolerance for enteric feeding. During PICU stay, the treatment approach was under the joint guidance of pediatric dermatology, neuropediatrics, and ophthalmology-daily topical application of fusidic acid and betamethasone and a 3-day course of methylprednisolone (10 mg/kg) followed by a 7-day course of prednisolone (2 mg/kg), with gradual improvement of the rash and oral mucosal lesions. The ocular pseudomembranes were removed every 2 days.
The regular antiepileptic therapy was suspended, only keeping clonazepam at the usual dose without worsening the seizures. He did an electroencephalogram (without sleep and with lots of movement artifacts) with no record of paroxysmal activity.
Serial laboratory evaluations showed no significant changes, and inflammatory parameters remained negative. Serologies for Epstein-Barr virus, Cytomegalovirus, herpes simplex virus types 1 and 2, parvovirus B19, Chlamydia trachomatis, and Mycoplasma pneumonia evidenced previous infection; SARS-CoV-2 and Bartonella henselae didn’t show active infections. Discharged 34 days after the onset of the illness and only medicated with clonazepam 0.05 mg/kg.
He maintained a multidisciplinary follow-up in the ambulatory consultation, with the resumption of myoclonic absences approximately 2 weeks after discharge (about 10 episodes/day). The dose of clonazepam was duplicated (0.1 mg/kg), and 5 months later, he started a ketogenic diet with significant improvement in seizure control.
Epicutaneous patch tests performed with a reduced Portuguese/European Baseline series, an antiepileptic series, and ethosuximide (a drop of the gel contained in the capsule as is and diluted at 10% in petrolatum) showed a strongly positive reaction (2+) to ethosuximide, whereas no reaction was observed on controls8. A skin biopsy was not performed. A genetic study was also carried out, and a variant of uncertain significance was identified in the POLG gene in heterozygosity, probably pathogenic. In order to study the genetic susceptibility for SJS, he’s currently awaiting results of the segregation of clinically relevant variants in parents.
Discussion
The diseases SJS/TEN are severe mucocutaneous adverse reactions, and although these conditions are commonly triggered by drugs (risk limited to the first 8 weeks of treatment), they can also have an infectious cause (such as Mycoplasma pneumonia, herpes simplex virus, and Epstein-Barr virus) or be caused by immunization, especially in children4,7.
Diagnostic criteria for SJS/TEN are not consensual, so histologic findings have low specificity. Due to these limitations, the diagnosis of SJS/TEN is based on the following clinical characteristics:
− Suggestive history of exposure to a new drug, 1-3 weeks (average 14 days) before the onset of the symptoms. A new contact with the drug may result in the reappearance of symptoms in < 48 hours-in our case, ethosuximide was started 9 days before the onset of the illness.
− A prodrome of acute-onset febrile illness and malaise-the patient started symptoms 18 days before the hospitalization that led to the diagnosis.
− Painful and rapidly progressive rash described by erythematous macules (coalescing and with the purpuric center), atypical targetoid lesions, or diffuse erythema, all of them developing to vesicles and soft bullae, as shown in Fig. 1.
− Positive Nikolsky and/or Asboe-Hansen signs.
− Oral, ocular, and/or urogenital mucositis with painful and hemorrhagic erosions. In our case, the child presented with cheilitis (Fig. 1C) and ocular pseudomembranes.
− Variable degree of necrosis and epidermis detachment.
Many drugs, including ASD, can cause adverse skin reactions, appearing in approximately 2-3% of prescriptions of a new ASD and being the most common reason for drug discontinuation. Although approximately 95% of adverse skin reactions are mild, such as morbilliform/maculopapular rash or urticaria and/or angioedema, they can occasionally be severe and potentially fatal6,7. According to a Japanese study5 that investigated the characteristics of SJS and TEN associated with ASDs in pediatric patients using a spontaneously reported adverse drug events database, severe cutaneous reactions were associated with multiple ASD. It may be important to avoid polymedication in order to minimize the risk of SJS/TEN during the treatment of children with ASD.
In the literature, there are few reported cases of SJS/TEN associated with ethosuximide, an ASD used mainly to control absence seizures5-7. When there is a suspicion of this diagnosis, the identification of the causative drug is essential because its early withdrawal can improve the prognosis7. In addition, skin patch tests can be helpful confirming allergy to ethosuximide as it helps to prevent re-exposure in patients recovering from SJS.
The main principles of supportive treatment include wound care, rehydration and electrolyte replacement, nutritional support and temperature, pain, and superinfections control, although prophylactic systemic antibiotics are not recommended. Ocular affection requires urgent care to reduce the risk of permanent ocular sequelae2.
In addition to supportive care, there are no universally accepted adjuvant therapies for SJS/TEN. Several immunosuppressive or immunomodulatory therapies have been used in clinical practice, including systemic corticosteroids, IVIG, cyclosporine, plasmapheresis, and anti-tumor necrosis factor monoclonal antibodies. None of these therapies have been successfully studied in randomized trials, but there is growing evidence that cyclosporine can slow the progression of SJS/TEN.
The use of systemic corticosteroids remains controversial9-11. A large European multicenter study10 and a meta-analysis11 suggest that a short course of moderate to high systemic corticosteroid therapy (e.g., prednisone 1-2 mg/kg daily for 3-5 days) may have a beneficial effect if administered within the first 24-48 hours of symptoms. Two pediatric cases described in the literature of SSJ induced by ethosuximide on children at the same age7 suggested that the therapeutic regimen of corticosteroids and/or IVIG may be effective in treating it, especially in the early stage of illness, as well as the clinical course of our patient.
The diseases SJS/TEN are rare, and the identification of the causative drug is crucial. Its discontinuation should be the first measure to be adopted when the diagnosis is established. In addition to supportive treatment, it’s difficult to establish an optimal treatment strategy due to the lack of well-designed clinical trials on outcomes.














