Introduction
Cutaneous drug reactions (CDRs) are a common reason for dermatologic consultation, with a clinical spectrum that ranges from self-limited and benign dermatosis to life-threatening conditions.
LDE has classically been associated with anti- hypertensive drugs (angiotensin-converting enzyme -inhibitors, β-blockers, and thiazides), gold, and penicillamine1,2 but over the years, the list of implicated agents has been growing.
A pruriginous rash composed of erythematous scaly papules and plaques usually distributed in the trunk and extremities is the most common presentation. Sometimes, lesions can resemble inflammatory dermatosis like psoriasis or eczema, may follow a photo-distributed pattern3 and present after a long latent period.
Clinical case
We report the case of a 63-year-old woman with a past medical history of hypertension treated with olmesartan for over 10 years, who developed gastritis and started treatment with esomeprazole 40 mg/day.
Around 2 weeks later, the patient developed a pruriginous disseminated dermatosis composed of violaceous polygonal flat-topped papules affecting the flexural aspects of the upper and lower limbs, abdominal flanks, and the lumbar and sacral regions (Figures 1 A and B). The remaining physical examination was unremarkable. Laboratory workup, including hemogram, ionogram, liver function, and renal function, were normal and anti-hepatitis C virus antibodies, venereal disease research laboratory test, and treponema pallidum haemagglutination test were negative.
A punch biopsy of an abdominal papule revealed irregular epidermal hyperplasia, hypergranulosis, apoptotic keratinocytes, areas of focal parakeratosis, and a dense band-like lymphocytic infiltrate in the upper dermis (Figure 2).
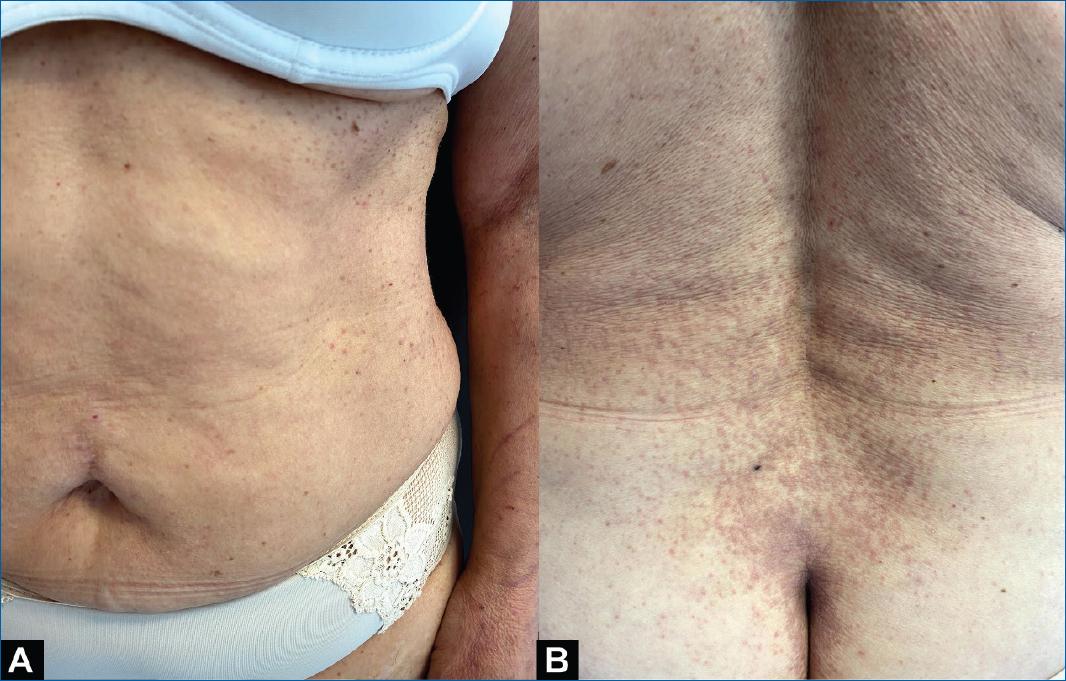
Figure 1 Lichenoid papules affecting the abdominal region (A). Close-up of lumbar and sacral lesions (B).

Figure 2 Histopathologic findings of a biopsy of an abdominal papule (hematoxyline-eosin staining, 100×).
A diagnosis of drug-induced lichen planus (LP) was made. The patient discontinued esomeprazole and was treated with medium-potency topical corticosteroids and emollient twice daily for 1 month. At the 2-month follow-up, most lesions had regressed with postinflammatory hyperpigmentation, and there was no recurrence at 6-month follow-up.
Discussion
Adverse drug reactions frequently involve the skin and follow, in most cases, benign courses. LDEs are relatively uncommon4 (unlike maculopapular rashes) and usually present in adults (median age 57-66 years)5.
The pathophysiology of LDE hasn’t been fully elucidated, and it is thought to differ, at least partially, from LP. Regardless, T8+ cells and granzyme B appear to be central key factors in its development1,6.
Differential diagnosis includes LP, subacute lupus erythematosus, psoriasis, eczema, secondary syphilis, and keratosis lichenoid chronica.
Differentiation from LP can be difficult but is crucial, as discontinuation of the inciting drug leads, in most cases, to the resolution of lesions (it is noteworthy, however, that some patients maintain symptoms even after drug removal)1. Clinically, classical sites of LP lesions, such as the flexural aspects of the limbs and mucosa, are less commonly affected in LDE and Wickham striae are frequently not found in the latter1,3. Histopathologically, even though there are several common features between these two entities, LDE often presents with eosinophils, focal parakeratosis, and focal interruption of the granular layer6. In this patient, however, eosinophils were not found on the skin biopsy, which, in itself, does not exclude the diagnosis of LDE.
Time to develop lesions after drug initiation differs between class types and is highly variable, ranging from weeks to several months or even years1,3.
In this case, clinical and histopathological findings compatible with LDE, temporal association with esomeprazole intake, resolution of symptoms, and lack of recurrence after drug withdrawal favor the diagnosis of esomeprazole-induced LP.
Proton-pump inhibitors (PPI) are one of the most commonly prescribed drug classes, and there are several published reports of cutaneous reactions associated with its use. These range from immediate immunoglobulin E mediated reactions (urticaria and anaphylaxis) to delayed-type hypersensitivity “Stevens-Johnson/toxic epidermal necrolysis, drug reaction with eosinophilia and systemic symptoms, fixed drug eruption, and drug-induced subacute lupus erythematosus, among others”4,7. There are three published case reports of PPI-induced LP2,4,8. Two patients were older males (median age 79.5 years)2,8, one of whom developed LDE to several PPIs8. The remaining case was a 2-year-old girl treated with esomeprazole4. Resolution of symptoms with drug withdrawal was reported in two of these cases4,8.
Being a relatively uncommon clinical entity, studies regarding the best clinical approach to treatment are lacking. Drug discontinuation is central to resolution, and, other than that, topical and systemic corticosteroids are the mainstream treatment1,2,4.
We report this case to highlight the importance of considering PPI as the culprit drug in similar clinical situations, as PPI-LDE is a rare entity.














