Introduction
Allergic contact dermatitis (ACD) to enemas is infrequently described in the literature. The greater likelihood of mucosal hypersensitivity, especially in cases of compromised integrity such as inflammatory bowel disease (IBD), makes enemas a potential source of sensitization and allergic reactions1,2. Since enemas are commonly used to treat IBD affecting the distal colon and rectum, it is believed that ACD is underreported, as symptoms may be mild and non-specific1.
Concerning ACD to topical medication affecting the anogenital area, a study from Gilissen et al. showed 9% positivity of patch tests to topical drug preparations, with local anesthetics and corticosteroids being the most frequent sensitizing active principles3.
Clinical case
A 55-year-old woman, without previous history of atopy, was under gastroenterology (GE) surveillance since 2019 for non-specific proctitis. The patient had been previously medicated with oral mesalazine, ciprofloxacin, and budesonide rectal foam. At the last GE appointment, daily rectal enemas of budesonide were prescribed. However, 1 day after the first application, erythematous, pruritic plaques emerged in the perineal region and inner thighs (Fig. 1). The patient was observed in the emergency department and was ordered to stop the enemas. She was medicated with oral bilastine and topical betamethasone and clotrimazole with the resolution of the skin eruption in 1 week.
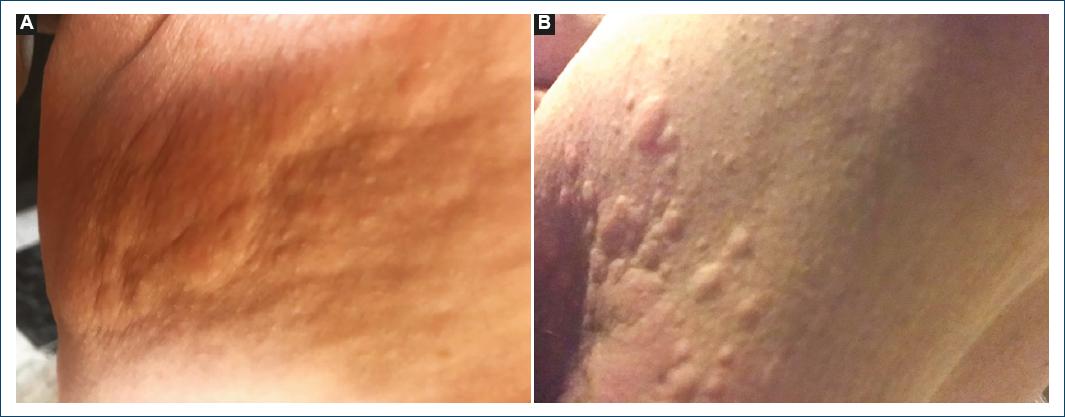
Figure 1 A and B: erythematous papules and plaques in inner ties after first budesonide-containing enema.
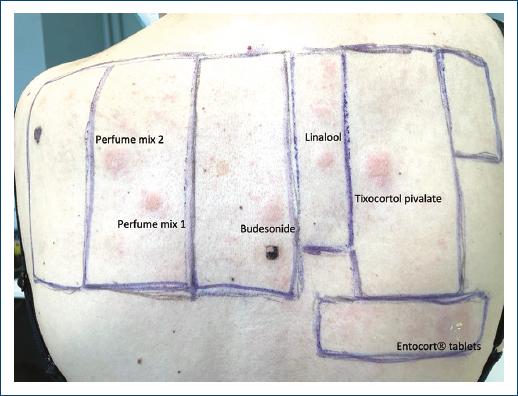
To clarify the cause of the skin eruption, the patient was referred to the dermatology department. Patch tests were performed using the European/Portuguese baseline series (Chemotechnique Diagnostics, Vellinge, Sweden), the corticosteroids series (Chemotechnique Diagnostics, Vellinge, Sweden), the dispersible tablets of budesonide and diluent solution (Entocort®) used in the enema and a moisturizing cream used by the patient (Odette®). Test readings at 72 h and 7 days revealed positivity for budesonide 0.1% (+), tixocortol pivalate 0.1% (++), the budesonide dispersible tablets used in enemas (+), perfume mixture 1-8% pet (+) and 2-14% pet (+) and hydroperoxides of linalool 1% (+) (Fig. 2). No lesions have recurred after the eviction of the identified allergens.
Discussion
This case illustrates an ACD to a budesonide-containing enema, with the budesonide foam acting as the likely source of prior sensitization.
Topical corticosteroids (CS) are a relatively common cause of ACD, with a reported positivity in patch testing ranging from 0.5% to 6%1. Allergy to CS in nasal sprays and inhalers used to treat asthma or rhinitis is well documented4. Surprisingly, there are fewer reports of CS’s ACD due to enemas, which possibly relates to the unspecific manifestations of allergic reactions in the anogenital area, sometimes misinterpreted as exacerbations of the previous inflammatory disease1.
Additional diagnostic obstacles for CS contact allergy include frequent false-negative reactions, as CS tends to suppress a positive patch test reaction and delayed positive reactions, as up to 30% of reactions may be missed if a day 7 reading is not performed1.
It has been postulated that, similarly to damaged skin, inflammation of the distal bowel may increase rectal CS’ penetration and facilitate sensitization2. In fact, Malik et al. showed a 9% (four out of 44 patients) prevalence of CS allergy among IBD patients; however, the authors admitted an eventual selection bias which hampers data extrapolation to the entire IBD population1.
The most used patch test allergens of CS allergy are tixocortol pivalate and budesonide as these are considered markers of steroid allergy1,2. By testing both, 90% of corticosteroid-allergic subjects are detected in baseline series1,2.
According to the new classification of CS by allergenic groups provided by Baeck et al., budesonide and tixocortol pivalate belong to the same group (group 1: the nonmethylated, most often nonhalogenated molecules)5. This study further classifies patients allergic to CS into two different subgroups according to the mechanism of immune recognition: patients who react to molecules from one unique group determined by CS molecular charge and patients who may react to the entire spectrum of CS determined by CS molecular structure5. Facing a patient with CS allergy, it is important to discern these two subgroups to determine the individual sensitization/tolerance profile5,6. In this case, one can assume the patient only reacts to molecules from group 1 since the lesions improved under topical betamethasone which belongs to group 3.
ACD to budesonide-containing enema is especially relevant since it is the topical drug that most frequently causes systemic allergic dermatitis7. The anorectal mucosa constitutes a relevant route of systemic absorption and its exposure to an allergen can trigger a reaction with variable clinical severity7.