INTRODUCTION
Soil reference values (SRV) are threshold concentrations of pollutants in soil that, when attained, an effect on the terrestrial ecosystem is expected. These values are generic screening standards used majorly to evaluate the potential toxicity of specific contaminants in the soils, providing valid and specific information about the impacts on the terrestrial biota when exposed (Friday, 1999; Pereira et al., 2018).
Toxicity data for plants, invertebrates, soil microbial activity, and sometimes mammals and birds, or even background concentrations obtained from natural soils, are frequently used to derive SRV for environmental risk assessment (Caetano et al., 2016; Pereira et al., 2018). The effects of the contaminant on the soil biota are tested at different endpoints and organisms. Then probabilistic or deterministic methods are applied for the estimations of the reference values for the pollutant, following different standard guidance documents, such are the European Commission Technical Guidance Document on Risk Assessment (European Commission, 2003), United States Environmental Protection Agency or the United States Environmental Protection Agency (USEPA), and the methods proposed by The Netherlands (Swartjes et al., 2012).
In Portugal, derivation of SRV started with a cambisol, which is the most common soil category in the country's north and center. For this kind of soil, SRV for copper, cadmium, and uranium have already been suggested (Caetano et al., 2016). The major objective of this work is to generate an ecotoxicological dataset using a specific type of Portuguese natural soil (regosol) for the derivation of soil reference values for arsenic (As). The regosol is the dominant type of soil at Estarreja region (center of Portugal), where historical problems of soil contamination were caused by the Estarreja Chemical Complex. Obtaining regional SRVs of the most concerning contaminants in the region, as for example As, to be used in for first-tier soil risk assessment purposes is of utmost importance for a site-specific evaluation.
MATERIAL AND METHODS
Based on the area's geological maps and historical soil uses, a representative regosol soil from Estarreja was collected from three different sites (40°45'47.5"N 8°35'49.6"W) randomly and mixed thoroughly to obtain a composite sample, as a true representative of a regosol from that area. Soil samples were collected (0-10 cm depth), labelled, transported, processed adequately (2 mm sieve was used for the enzyme and aquatic toxicity assays and 4 mm for the assays with terrestrial invertebrates and plant assays), and stored depending on the parameters to be analysed (e.g., oven/air-dried for physical-chemical analysis).
The soil was spiked with a range of concentrations, defined based on the Effect concentration (ECx) sampling design (12 concentrations with minimum of 3 replicates per concentrations) and left for 48 hours. The concentrations tested were 0, 1.86, 3.25, 5.68, 9.95, 17.41, 30.46, 54.31, 93.29, 163.27, 285.71 and 500 mg of As kg-1 of soildw respectively. After 48 hours, the spiked soil samples were used to perform a battery of terrestrial ecotoxicological tests with Eisenia fetida and Folsomia candida to assess the effects on the reproduction of terrestrial invertebrates following the OECD 232 and 222 criteria, the seedling emergence and growth of terrestrial plants (at least two monocotyledonous and two dicotyledonous species) following the OECD 208 protocol. In our case, we used Avena sativa, Triticum aestivum, Lactuca sativa and Solanum lycopersicum respectively. The contaminated soils were also brought in contact with deionized water containing 0.001 mol/l of CaCl2 solution (in a liquid to solid ratio of 4:1 l/kg) and agitated for 24 hours until near equilibrium between liquid and solid phase was achieved. After 24 hours, the samples were taken to the centrifuged for 20 min and then the supernatant was collected, sieved with a 0.45 μm syringe filters and then used for the aquatic toxicity assays with Daphnia magna (immobilization test following OECD 202), Raphidocelis subcapitata and Lemna minor (Growth inhibition tests following OECD 201 and 221) and Allivibrio fischeri (following the Microtox® model 500 Toxicity Analyzer (Modern Water, New Castle, DE) with the 81.9% basic test protocol.
A sample of fresh soil was also contaminated with the range of concentrations of As as stated previously and incubated for 30 days under a well-controlled photoperiod and temperature conditions, for microbial parameters. After 30 days, the contaminated soil was used to assess the activity of several soil microbial enzymes (dehydrogenases, cellulase (Schinner & von Mersi, 1990; Schinner et al., 1996), arylsulfatase (Tabatabai & Bremner, 1970; Schinner et al., 1996), urease (Schinner et al., 1996; Kandeler & Gerber, 1998), phosphatases, potential nitrification and nitrogen mineralisation (Keeney, 1983; Schinner et al., 1996).
A one-way analysis of variance test was used to test for a significant effect of the pollutant concentrations on each of the tested species/parameters. After all the ANOVA assumptions had been met, Dunnett's post-hoc test was employed to determine which concentrations differed significantly from the control and the LOEC (low observed effect concentration) and NOEC (no observed effect concentration) values obtained. The EC50, EC20 and EC10 values were calculated using the DRC package in R and compared with the STATISTICA® 7.0 software (StatSoft, Inc., Tulsa, OK, USA). All the estimations were recorded at a 95% confidence interval and alpha 0.05.
The USEPA species sensitivity distribution software and the ssdtool package in R were used to estimate the percentage hazard concentration (HCp), and the protection level was determined and proposed as the SRV for As in regosol.
RESULTS AND DISCUSSION
Arsenic was very toxic for all the plant species tested, with total inhibition noted at ≥ 281 mg As kg-1 soildw. However, L. sativa had a better germination percentage than H. vulgare, but the dry mass of H. vulgare proved to be a more sensitive endpoint amongst the parameters tested for plants. Figure 1 shows the effect of As on the growth of one of the terrestrial plants (L. sativa).
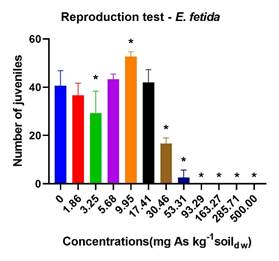
For the invertebrates, As significantly inhibited the reproduction of E. fetida (p<0.01) and F. candida (p<0.01) as the concentration increases, with a total inhibition noted at ≥ 93.29 mg As kg-1 soildw as shown in figure 2. The NOEC and LOEC values recorded were 1.86 mg As kg-1 soildw and 3.35 mg As kg-1 soildw and 5.68mg As kg-1 soildw and 9.95mg As kg-1 soildw, respectively, for these organisms.
For the microbial parameters, As inhibited the activity of acid phosphatase as the concentration increases with concentrations ≥ 93.95 mg As kg-1 soildw being significantly different from the control. Furthermore, As inhibited dehydrogenase activity with the activity at concentrations ≥ 30.46 mg As kg-1 soildw being significantly different from the control.
For the aquatic organisms, As was extremely toxic to D. magna, with total immobilization being noted in the elutriate prepared from soils with concentrations above 17.41 mg As kg-1 soildw.
CONCLUSIONS
The ecotoxicological data gathered in this study showed that As has an impact on the soil and aquatic community, even at the lowest concentrations tested, when the organisms are exposed through a regosol
The estimated Effect Concentrations (ECx) that were calculated from each of the experiments were used to derive the reference values.
This was achieved by fitting the most sensitive endpoints from each of the assays to a mathematical model using the USEPA SSD software to derive a HC5 for EC10, EC20 and EC50. Based on the data collected, an SRV of 2.80 mg As kg-1 soildw, with R2 of 0.96 was proposed.
Other assays are currently running, and the data would be used to generate an SRV that genuinely represents the ecological reality of the study location, which is related to agricultural soils.