Introduction
Vascular malformations (VMs) are a heterogeneous group of rare vascular development disorders with historically few treatment options. Recent studies have shown the potential benefit of mammalian target rapamycin (mTOR) inhibitors-such as sirolimus-in their treatment, particularly in low-flow malformations.1 However, reports on their efficacy in high-flow malformations are scarce, and the results are controversial.2-4 We report the successful case of a patient with a combined malformation with an arteriovenous component who benefited from treatment with sirolimus.
Report
A three-year-old female patient was referred to our center with a diffuse and progressive vascular malformation on her right leg, difficult to classify according to the International Society for the Study of Vascular Anomalies (ISSVA) classification, with disabling pain, limb deformity, and walking impairment.
Initial magnetic resonance imaging (MRI) showed a diffuse malformation in the deep subcutaneous tissue without muscle or bone involvement, measuring 18cm on the largest axis. Two feeding arteries - one arising from the tibioperoneal trunk and another from the popliteal artery - communicate with both the popliteal and great saphenous vein (Figure 1).
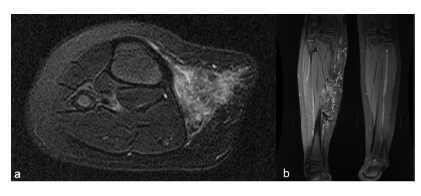
Figure 1 Magnetic resonance imaging showing an extensive diffuse malformation on the deep subcutaneous tissue of the medial side of the right leg. Axial (a) and coronal views (b).
Conservative treatment with a grade II compression stocking and daily physical therapy was started and maintained for several years, resulting in minor improvements. The patient remained symptomatic, and progressive worsening was noted over time. At age nine, it was decided to discuss the case with an international reference center in vascular malformations (Royal Free Hospital), and the malformation was then classified as a diffuse vascular malformation with involvement of contiguous structures without defined borders, with both a low and high-flow component. The high-flow component started behaving like a type IV Yakes arteriovenous malformation over time (Figures 2 and 3).
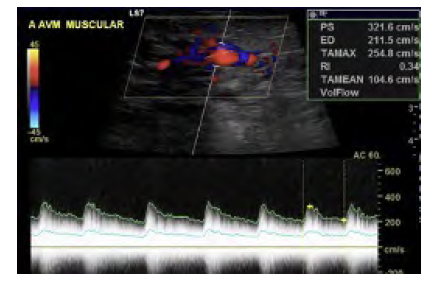
Figure 2 Doppler ultrasound of the vascular malformation. Low-resistance flow in the involved arteries and arterialization of flow in the involved veins is noted, with high peak velocities in both channels.
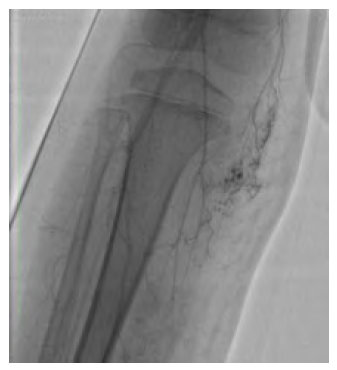
Figure 3 Diagnostic angiography of the vascular malformation. Scarce but diffuse high-flow nidus is noted.
Considering the main low-flow component, it was decided to institute sirolimus therapy in collaboration with the pediatric specialists from our multidisciplinary team (hematology/oncology and immunodeficiency specialists). Thus, long-term therapy with sirolimus (0.8 mg/m2 twice daily, progressively adjusted according to the patient's height and weight changes) was instituted when the patient was nine. This prolonged therapy resulted in complete remission of pain, recovery of walking ability, and partial recovery of the valgus of the knee. No side effects were reported.
With 38 months of follow-up and therapy, the 13-year-old remains pain-free and has an improved quality of life despite the absence of imagological improvement.
Discussion
Sirolimus, a mTOR inhibitor, was first used in the management of vascular malformations due to its antiangiogenic properties in 2010.5 mTOR is a serine/threonine kinase regulated by phosphoinositide 3 kinase (PI3K) and protein kinase B (Akt). The PI3K/AKT/mTOR pathway is an important intracellular signaling pathway in the regulation of cell growth and proliferation. Heightened mTOR signaling leads to an increase in the expression of the vascular endothelial growth factor (VEGF), resulting in angiogenesis and lymphangiogenesis augmentation; thus, an inhibition of the PI3K/AKT/mTOR pathway leads to a blockage in cell proliferation and angiogenesis.1,6 This pathway mainly induces low-flow vascular malformations, such as venous or lymphatic malformations. High-flow malformations, including arteriovenous malformations, are primarily associated with another pathway - the RAS/MEK/ERK pathway.6
Therefore, the benefit of sirolimus is highly variable according to the type of VM (high-flow vs. low-flow) and patient genotype. Low-flow malformations, overgrowth syndromes, and other VMs associated with genetically associated upregulation of the mTOR pathway respond better to sirolimus.1 Both pathways are related via RAS, which motivated the investigation of the benefit of sirolimus in high-flow VMs, leading to reports showing conflicting results.2-4,7
Individual patient characteristics should be considered when deciding whether to initiate sirolimus, including type, location, size, and dimension growth of the VMs.2 Some reports show evidence of benefit with sirolimus therapy, with a size reduction of the deforming mass and improvement of swelling, pain, and functional impairment.7-8 A recent study by Triana et al. included 122 patients with high-flow malformations, with the most described symptoms consisting of pain (84/122 patients), deformity (65/122), and inflammation/infection (62/122). Overall response was positive, with 90.8% of patients reporting benefit with treatment. Nevertheless, 11.6% of patients reported stability, and 1.5% of patients reported progression of the malformation during therapy.8 In our case, the patient reported complete remission of pain and functional impairment despite no improvement in terms of limb deformity or on imagological follow-up.
Presently, there is still no consensus on when to initiate treatment with sirolimus in patients with VMs or on how long to maintain it for, although reports point towards a better and faster response when sirolimus is started at a lower age, particularly in patients <5 years of age.8 This may also help reduce morbidities associated with VMs, avoid future invasive interventions, and improve prognosis.1 Response time after the start of therapy is highly variable, with some patients showing significant response after 2-4 months while others need over two years.7-8 Because of this, guidance relating to treatment duration is still lacking. In our case, the patient reported significant improvement in pain and functional impairment as early as four weeks after starting sirolimus. Due to overall continued improvement, it was decided to maintain long-term treatment as long as the patient remains clinically stable. We also opted not to perform a more invasive concomitant treatment, such as sclerotherapy or VM surgical resection, considering early and significant improvement with sirolimus.
As most patients are of pediatric age and require long-term treatment, side effects are an essential concern.2 The most common side effects are mucositis, dyslipidemia, leukopenia, gastrointestinal symptoms, rash, and infectious complications, including cases of fatal Pneumocystis jirovecii pneumonia.2-3 For this reason, some authors advocate using antibiotic prophylaxis with trimethoprim-sulfamethoxazole.2 No side effects were reported in our patient, and no prophylaxis was used.
In conclusion, despite the available evidence for mTOR inhibitors being mainly for low-flow malformations, it may be a safe and effective option for selected high-flow malformations when intervention is not possible or carries significant morbidity risks. Some studies showed a reduction in the size of the malformation with treatment; however, this was not the case in our patient, notwithstanding the clear improvement in quality of life for over three years without other intervention. Future investigation is needed to standardize practice, particularly in ascertaining which specific disease phenotypes and/or genotypes are the best responders, the ideal timing for starting therapy, and the duration of treatment.














