Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Corrosão e Protecção de Materiais
versão impressa ISSN 0870-1164
Corros. Prot. Mater. vol.33 no.4 Lisboa dez. 2014
ARTIGO
Atmospheric corrosion in museum context – the case of the treasure room from the national archaeology museum, Lisbon
Corrosão atmosférica em contexto museológico – O caso da sala do tesouro do museu nacional de arqueologia, Lisboa
I. Tissot*1,2, M. Tissot2 and M. F. Guerra1,3
(1) LIBPhys-UNL, Department of Physics, Faculty of Science and Technology, New University of Lisbon, 2829-516, Caparica, Lisbon
(2) Archeofactu, Rua do Cerrado das Oliveiras, 14, 2º Dtº, 2610-035 Alfragide, Amadora, e-mail: mathias.tissot@archeofactu.pt
(3) CNRS-UMR 8096 ArchAm, 92023 Nanterre, France, e-mails: maria-filomena.guerra@mae.u-paris10.fr
(*) A quem a correspondência deve ser dirigida, e-mail: isabel.tissot@archeofactu.pt
ABSTRACT
The gold jewellery collection exhibited in the Treasure Room of the National Archaeology Museum in Lisbon, Portugal, developed a severe surface corrosion. In order to approach the corrosion mechanisms, the state of conservation of the objects and the exhibition conditions were characterised. The temperature, the relative air humidity and the light were measured. The pollutants and the exhibition materials of the showcases were identified. The relation between the exhibition conditions and the corrosion development was search.
Keywords: Atmospheric Corrosion, Museum Exhibition, Gold Alloy Corrosion, Jewellery
RESUMO
A colecção de ourivesaria arcaica exposta na Sala do Tesouro do Museu Nacional de Arqueologia em Lisboa, Portugal, apresenta um desenvolvimento acentuado de corrosão. Para o estudo dos mecanismos de corrosão foram caracterizados o estado de conservação dos objectos e as condições de exposição. A temperatura, a humidade relativa, a luz, os poluentes e os materiais das vitrinas foram identificados. Foi investigada a relação entre as condições de exposição e o desenvolvimento de corrosão.
Palavras-chave: Corrosão Atmosférica, Condições de Exposição, Corrosão de Ligas de Ouro, Ourivesaria
1. INTRODUCTION
1.1. The surface corrosion of gold objects and the case of National
The preservation of jewellery collections in museum context has been raising in the last 10 years several questions [1-3]. Gold alloys are vulnerable to tarnishing and their corrosion resistance decreases with the increase of the alloying elements, Ag and Cu [4]. This phenomenon occurs when the objects are exposed to particularly high humidity sulphur-containing atmospheres.
The corrosion of gold alloys is characterized by the development of thin tarnish layers (their thickness may attain values below 1 µm). The colour of the tarnished surface ranges from yellow (very similar to the colour of the gold-base alloy) to dark grey with a multihued iridescent effect [5], causing aesthetical spots and extended zones on the objects surface.
The majority of the objects displayed in the exhibition Treasures of Portuguese Archaeology at the NAM, in Lisbon, are corroded. Figure 1 illustrates the quite light yellow surface corrosion on the fragment of torc Au 283 (this torc from Alentejo was produced with an alloy containing 80,6% Au, 19,3% Ag, 0,1% Cu) and the reddish corrosion on the archers armband Au 51 from Vila Nova de Cerveira (this armband was produced with an alloy containing 86,2% Au, 13,5% Ag, 0,3% Cu). The very slight surface alteration of the fragment of torc Au 283 contrasts with the high level of corrosion on the archers armband. The reverse surface of the latter shows however a lower corrosion development than the obverse. The different corrosion rates could be connected to distinct surface polishing processes.

Other objects either displayed in the Treasure room show preferential corrosion due to ancient restorations or to thermic and mechanical stress induced by deformation during fabrication and during burial (Figure 2).
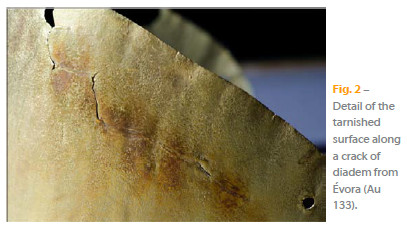
Beyond the aesthetically surface, the corrosion development has significant consequences on the state of conservation of the objects. The surface alteration requires restoration that involves, in general, mechanical cleaning operations, which remove material. The repeated cleaning causes wear which can attain, in certain cases, a material loss of 1 to 3% of the object's weight in a period of 200 to 300 years [6]. The removal of a surface layer may also erase the toolmarks and the usewear marks characteristic of the objects production and function.
The objects conservation can only be guaranteed when the corrosion products are identified and the atmospheric conditions of the conservation room environment are well controlled [7].
Because they remain in a very thin layer, the corrosion products of the gold alloys are difficult to identify. Although only a few studies on gold corrosion were carried out, all the publications [8-11] pointed out Ag2S as the main component of the corrosion layer, and refer the presence in the corroded layer of diverse compounds such as Ag3AuS2, AgAuS, Ag2SO2 and Cu2S. However, the chemical reaction undergone by silver-gold sulphides is still insufficiently studied preventing the establishment of a direct relation between the development of sulphides and corrosion [12].
1.2 The NAM and theTreasure Room climate
The main factor of the corrosion development is the location of the museums (climate characteristics), the nature of the exhibition room and the showcases materials, namely the type of wood, fibres, etc. [13].
The NAM is located at the Virgin of Belém Monastery, generally known as Jerónimos Monastery. This historical building, located in the western area of Lisbon, is implemented at 400 m from the north shore of the Tagus River, at 8 km from the river mouth. The city of Lisbon has a Mediterranean macroclimate, characterized by a summer with high temperatures and dry air and with the annual precipitation concentrated between October and April. This classic Mediterranean climate is however modified by the regional topography, particularly by the nearness of the Atlantic Ocean and the Tagus river. The average of the monthly temperature varies regularly throughout the year, being the average annual temperature of 17ºC. The annual relative air humidity (RH) average is high, ranging between 72% and 79%, which turns the Lisbon with RH levels climate similar to the moderated tropical regions, as for example, the city of São Paulo, Brazil [14].
Generally, during winter and autumn the RH average values are above 80%, while during spring and summer they range between 60% and 70%. These very high RH levels raise the risk of degradation in the case of metallic objects.
The high RH average annual values led to the characterisation of the environmental behaviour of the Treasure room in order to verify the influence of the outdoor climate. We remark that the room was designed as a box container at the northeast gallery on the ground floor of the NAM with an air conditioning system. This system only allows the temperature control, and it is programmed to maintain the room temperature at 19ºC.
This work presents the characterization of the room exhibition conditions (temperature, RH, lightning, pollutants and showcase materials) as a first approach to the study of atmospheric corrosion of the gold jewellery.
2. METHODS AND INSTRUMENTATION
The experimental work was carried out by using several science-based techniques that give information on the gold objects composition and on the indoor environment.
The analytical protocol developed considered the measurement of the outdoor and indoor temperature and RH by placing dataloggers onsite during twenty-two months. The identification of the room pollutants was carried out by corrosivity tests using different metallic coupons that were analysed by different ex-situ analytical techniques. The identification of the gold alloy corrosion considered the fragility and the rarity of the objects. The information was obtained in-situ with non-destructive techniques and by recreating both the alloys and the environment conditions for ex-situ analysis, which does not require moving the object to a laboratory.
Temperature, RH, light and UV: Outdoor and indoor temperature and RH were measured by using data loggers from Testo model, Testostor 175 Logger. Light and UV were measured by using a lux meter Lutron LX-101 and a UV meter Elsec Type 763 UV Monitor.
Showcase fabrics: The blue and green showcase fabrics were identified with a Philips XL 30 ESEM operated at 10 kV.
Corrosivity tests: Accelerated corrosion tests based on the Oddy test method [15,16] were carried out to test the corrosivity of the showcase materials. Samples of wood, pressed wood, glues, and fabrics were placed in glass flasks together with silver, copper and lead coupons (these materials are sensitive to degradation) sealed after adding a small quantity of deionised water to create a high RH atmosphere. One control flask contains only the coupons and the deionised water. The flasks were placed during 28 days into a heated oven at 60ºC (Figure 3).

The gold alloy coupons accelerated ageing test was carried out by adjusting the previous protocol using the blue tissue as degradation source and placing the gold alloy coupons inside the glass flask.
Gold alloy composition: The identification of the gold alloys was carried out in-situ with mobile EDXRF equipment that comprises an Oxford Instruments Eclipse IV X-ray source (45 kV, 50 µA, 2.25 W max.) with an anode of Rh and a 250 µm thick Be window coupled to a XR- 100SDD Amptek X-ray detector and preamplifier. The accuracy of the quantitative results was validated by analysis of a set of five homemade gold standards.
Corrosion products: The corrosion products were identified with a Bruker-Siemens D5000 µ-XRD equipped with a Cu anode X-ray tube. The morphology of the corrosion products was characterized with a Philips XL 30 ESEM FEG (field emission electron source) operated at 20 kV and coupled to an EDX unit from EDAX.
3. RESULTS AND DISCUSSION
3.1 RH, temperature and light
Figures 4 and 5 show the typical behaviour of the indoor and outdoor RH and T, during approximately one month when the museum was opened to visitors. The Treasure room is permanently open during the opening hours of the museum: 8 hours per day, 6 days a week. Due to the air exchange, equivalent indoor and outdoor RH fluctuations could be observed. Inside the Treasure room the RH reached 80%. The temperature fluctuation is less pronounced, ranging from 17ºC to 22ºC. The maximum seasonal variations in T and RH are respectively of 4ºC and 26% during autumn/winter.
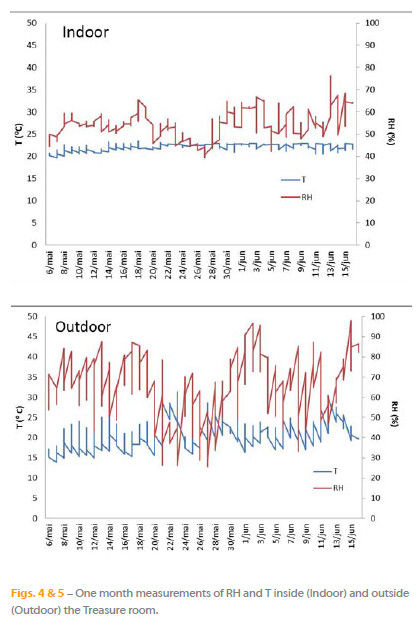
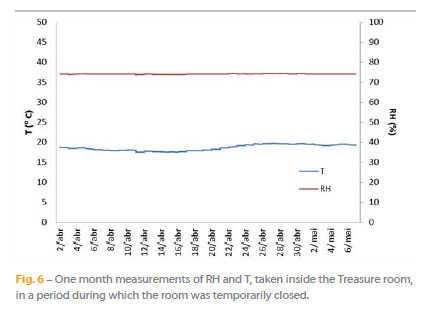
Figure 6 shows the HR and T values registered inside the Treasure Room when the room was temporarily closed to visitors. During this period no variation in T (19 °C) and RH (75%) could be observed, which allows assuming that the permanently opened access door is the main factor of the accentuated HR and T variation in Figures 4 and 5.
Fluorescent lamps lighten the room and the showcases. Each showcase has inside a set of fluorescent lamps. The UV emission values are below 45 µW/lumen and the illumination values range between 80 and 400 lux, in function of the distance from the light source. Most of the values are under 150 lux, which is the recommend limit in the case of metallic objects. [17]. Nevertheless, the measured values might accelerate the cover fabrics degradation, increasing the emanation of volatile organic compounds.
3.2 Constituent materials of the Treasure room
The main pollutant sources of degradation are the exhibition materials, which during their own degradation process release volatile organic compounds that gradually induce corrosion on the displayed objects [18].
The Treasure room is a box built inside the stone historical building. This box was made with wood and pressed wood (sometimes painted) with elements in steel. The showcases, in addition to wood and pressed wood, also contain aluminium, glass and 2 types of covering fabrics, one blue and another green (Figure 7).

Oddy tests were carried out for the pressed wood, the wood, the two fabrics and the glues used in the exhibition room. Only the fabrics induced corrosion on the silver coupons. Their analysis by XRD identified the presence of Ag2S. This corrosion product is in general associated to the presence of sulphur. The morphology of the fibres observed by SEM shows that both fabrics contain wool. The green fabric is made of wool and the blue fabrics contains wool and polyester fibres.
3.3 The pollutants identification
The identification of the pollutants was carried out by placing coupons of silver, copper and lead, inside and outside both the showcases and the room. Inside the showcases, the coupons were placed directly on the fabrics close to the lightening sources and in the room they were placed close to the exit of the air conditioning system.
When analysed by XRD, the lead coupons placed inside the room showed the presence of lead formate, Pb(CHO2)2 and hydrocerussite, Pb3(CO3)2(OH)2. These compounds are related to the present of volatile organic compounds, which can be attributed to the pressed wood. The silver coupons placed inside the showcases showed the presence of acanthite, Ag2S, while those placed outside the showcase showed the presence of chlorargyrite, AgCl. The silver coupon placed close to the exit of the air conditioning system revealed the presence of Ag2S and AgCl. The latter is expected in museum environments located near the sea, while the other silver sulphides could be explained by the presence of wool fabrics. It should be emphasised that sulphur based corrosion products are dominant in indoor environments (contrary to the chlorine based dominant corrosion products in outdoor environments) [19].The only copper coupon presenting corrosion, identified by XRD as cuprite, Cu2O, was the coupon placed outside the museum.
3.4. Atmospheric corrosion of the objects
Considering the results obtained for the room pollutants and the environmental conditions, we assumed that the sulphur released by the wool fabrics inside the showcases and the high HR inside the room are the main cause of the gold objects tarnishing.
Objects with pronounced corrosion development displayed in one showcase were selected to check this assumption. The objects were analysed for the composition of the alloys by EDXRF. Coupons made from similar alloys were fabricated by a goldsmith and submitted to accelerate ageing within a high S and RH atmosphere.
On the surface, the gold alloy coupons developed a visually similar tarnish to the objects. The corroded areas observed under the SEM correspond to porous structures made of aggregates of small rounded particles (Figure 8) that could be identified as sulphur compounds by XRD. Areas with a more developed corrosion show a complex structure that can be related to different corrosion products. The XRD analysis revealed the presence of AuAgS, Ag2S, and Cu3Au, but it must be noted that other crystalline compounds are present in the spectra. These compounds could not be identified, because the corrosion mechanisms of gold-base alloys are complex and not fully identified. The products to be identified by XRD must still be included in the compounds database of this technique.

4. CONCLUSIONS
It could be shown that the environment of the NAM Treasure room is characterised by high levels of humidity and sulphur. The main source of sulphur could be identified. In fact, and contrary to what was expected, the cover fabrics inside the showcases contain wool. The high RH can be explained by the permanently opened room door during the museum opening hours.
This room environment increases the corrosion rate of the gold objects. Gold alloy coupons, with similar composition to the objects, were produced to recreate, at the exhibition conditions, the corrosion developed on the objects. The tarnish developed on the surface of both the coupons and the objects was visually similar. The corrosion products on the coupons were identified by XRD and SEM. It could be shown the presence of AuAgS, Ag2S, and Cu3Au, even if some compounds also present in the corrosion layers could not be identified because of their absence from the XRD compound databases.
Based on this first approach to the atmospheric corrosion of gold alloy objects, a research on the corrosion mechanisms of these alloys will be established considering that this type of objects can hardly be moved from the exhibition rooms to a laboratory.
Acknowledgments
The research leading to the published results has received funding from the Portuguese FCT (Foundation for Science and Technology) under the AuCORRE project PTDC/HIS-HIS/114698/2009. I. Tissot acknowledges grant SFRH/BDE/51439/2011 from the Portuguese FCT.
REFERENCES
[1] D. Liang, C. Yang and N. Huang, Surf. Interface Anal., 43, 763 – 769 (2011). [ Links ]
[2] M. Griesser, R. Traum, K. E. Mayerhofer, K. Pipltis, R. Denk and H. Winter, Surf. Eng., 21, 385 -392 (2005). [ Links ]
[3] G. Gusmano, R. Montanari, S. Kaciulis, G. Montesper-Elli and R. Denk, Appl. Phys. A, 79, 205-211 (2004). [ Links ]
[4] K. Mayerhofer, K. Pipltis, R. Traum, M. Griesser and H. Hutter, Appl. Surf. Sci., 252, 133-138 (2005). [ Links ]
[5] M. F. Guerr and I. Tissot, Nucl. Instrum. Meth. Phys. Res. B, 306, 227-231 (2013). [ Links ]
[6] A. Lins and N. McMahon, (The inhibition of silver tarnishing) in Current Problems in the conservation of Metal Antiquities. 13th International Symposium on the Conservation and Restoration of Cultural Property, October, Tokyo, Japan (1993). [ Links ]
[7] V. Dagostino, F. R. DAmbrosio Alfano, B. I. Palella and G. Riccio (The museum environment: A protocol for evaluation of microclimatic conditions), Energ. Buildings (2014), in press. [ Links ]
[8] C. Liang, C. Yang and N. Huang, Surf. Interface Anal., 43, 763–769 (2011). [ Links ]
[9] D. Scott, Stud. Conserv., 28 (4), 194-203 (1983). [ Links ]
[10] J. H. Frant and D. Schorsch, Archeomaterials, 4, 133-152 (1990). [ Links ]
[11] E. Campo, E. Vera, J. Göllner, C. A. Ortiz and R. Lleras, Rev. LatinAm, Metal. Mater. S1(4), 657-661 (2009). [ Links ]
[12] G. A. Palyanova, Y. V. Seryotkin and K. A. Kokh, Dokl. Earth Sci., 436, 42–46 (2011). [ Links ]
[13] P. Brimblecombe, N. Blades, D. Camuffo, G. Sturaro, A. Valentino, K. Gysels, R. Van Grieken, H.-J. Busse, O. Kim, U. Ulrych and M. Wieser, Indoor Air, 9, 146–164 (1999). [ Links ]
[14] I. V. Aoki, T. C. Diamantino, M. J. F. Marques, I. F. Vasques and M. E. D. Taqueda, Corros. Prot. Mater., 26, 40-45 (2007). [ Links ]
[15] P. Hatchfield. Pollutants in the Museum Environment: Practical Strategies for Problem Solving in Design, Exhibition and Storage, Archetype Publications, London, UK (2002). [ Links ]
[16] J. Tétreault, Bulletin Technique nº 21, Ottawa, Institute Canadien de Conservation (1999). [ Links ]
[17] S. Michalski, (Light, ultraviolet and infrared) in Ten agents of deterioration. Ottawa: Government of Canada. Department of Canadian Heritage. Canadian Conservation Institute, (2010). http://www.cci-icc.gc.ca/resources-ressources/agentsofdeterioration-agentsdedeterioration/chap08-eng.aspx [ Links ]
[18] J. Tétreault, Display Materials: The Good, the Bad and the Ugly. Edinburgh, Scottish Society for Conservation and Restoration (SSCR) Exhibition and Conservation Preprint (1994). [ Links ]
[19] H. Lin, G. S. Frankel and W. H. Abbott, [ Links ] J. Electrochem. Soc., 160, C345-C355 (2013).
Artigo submetido em Dezembro de 2014 e aceite em Janeiro de 2015













