INTRODUCTION
Nitrogen fertilizers are a key component in the production of field crops. In late 2021, fertilizer prices began to raise going from 216 €/t to 355.5 €/t in the case of ammonium sulfate 21%, according to data from the Spanish Ministry of Agriculture, Fisheries and Food (MAPA, 2022). In this scenario, recovery of nutrients, such as N are of special relevance, even more from N rich livestock waste as manure or treated manure as digestate.
Recently, the gas-permeable membrane technology has been identified as one of the most energy and cost-effective technology for recovering N from waste (Beckinghausen et al., 2020).
The aim of this work was to use gas-permeable membrane technology to capture ammonia from digested slurry co-digested with orange peel residue, obtaining a liquid solution of ammonium sulfate that has been tested as a fertilizer in micropot trials, since it has been scarcely studied .
MATERIALS AND METHODS
Nitrogen recovery
A batch experiment was conducted in 2 L plastic jars (1.5 L digestate). The acid tank consisted of 500 mL Erlenmeyer flasks containing 100 mL of 0.5M H2SO4 (Panreac). A peristaltic pump (Pumpdrive 5001, Heidolph, Schwabach, Germany) was used to continuously recirculate the acidic solution through tubular membranes inside the digestate jars and back into the acid tank using a constant flow rate of 12 L d−1. The acid pH was adjusted to keep it below 2. Gas-permeable tubular membrane (48 cm long, 5.2 mm outer diameter) made of expanded polytetrafluoroethylene (e-PTFE) (Zeus Industrial Products Inc., Orangeburg, SC, USA) was used for NH3 capture. The membrane was submerged in the digestate. Low-rate aeration was used to increase digestate pH according to García-González et al. (2015). The airflow rate was 0.24 L-air L digestate−1 min−1 in the first two days and 0.13 L-air L digestate−1 min−1 to the end of the experiment and it was controlled using an airflow meter (Aalborg, Orangeburg, NY, USA). Ammonia was measured as total ammoniacal nitrogen (TAN).
Micropot trials
A micropot experiment with two plants of triticale (× Triticosecale Wittmack) in each pot as crop test was performed in a growth chamber, during 34 days. The soil used was a dystric Cambisol. The fertilization was done with a half-strength Hoagland solution with or without N, the last with the addition of the N recovered solution (Hoag and N-rec treatments respectively). These solutions were applied in every pots, according to the treatments, during the experiment (totaling 100 mg of N for each pot). A treatment without any fertilization was also done (W treatment). The N sources for the triticale were the mineral N from the Hoagland solution (Hoag) or the N recovered from the digestate (N-rec). After the end of the experiment, the plants of each treatment and repetition (four repetions/treatment) were cut and weight (fresh matter) and then were dry at 65 ºC and weight for dry matter. The total N was quantified in the triticale by the Kjeldahl procedure and the crop N uptake of each treatment was evaluated.
One-way ANOVA analyses were conducted to identify the effect of the treatments on the triticale biomass production and N uptake. Tukey's test was used to compare means at 0.05 probability level.
RESULTS AND DISCUSSION
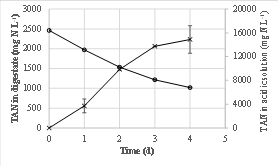
The aeration increased the pH of the digestate from 8.48 to up 9.48. The total ammoniacal nitrogen concentration in the digestate decreased from 2462 ± 24 to 1019 ± 12 mg N L-1 in four days of the experiment (Figure 1). Simultaneously, TAN concentration in the acid solution increased up to 14889 ± 2324 mg N L-1, that was more than six-fold higher than in raw digestate. Sixty-two percent of TAN removed from digestate was recovered in the acid solution. This value was the same than this obtained by García-González et al. (2016) when recovering ammonia from anaerobically digestate manure using gas-permeable membranes in a 32-day experiment. A percentage of the TAN removed (37.7%) was volatilized to the atmosphere as a consequence of the high pH achieved during the treatment.
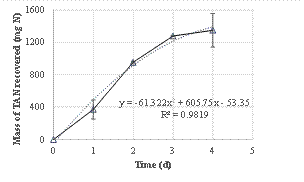
The TAN recovery was not linear and followed a 2nd-order curve, as shown in Figure 2, meaning that the TAN capture rate was higher during the first days, and decreased with time. The TAN recovery rate was 43.0 ± 6.6 g N per m2 of membrane and per day. This value is higher than the values obtained in previous batch studies with similar treatment time found in literature. For example, Vanotti et al. (2017) treated anaerobically digested swine wastewater containing 2350 mg N L-1 using submerged membranes plus low-rate aeration to recover ammonia and reported a TAN recovery rate of 25.1 g TAN m-2 d-1. Dube et al. (2016) tested the use of gas-permeable membranes for recovery ammonia from anaerobically digested swine manure and obtained TAN recovery efficiencies between 22.7 and 30.7 g N m-2 d-1.
The data obtained showed a N recovery of 62% from the digestate in four days, and a N recovery rate of 43.0 ± 6.6 g N per m2 of membrane and per day.
The results of the micropots trials testing the ammonium sulfate solution, showed that irrespective to the N source the fertilization with nitrogen increased significantly (p<0.01) compared with no N fertilization, being the N-rec the treatment with the highest biomass production (Fresh matter, Figure 3). However the tritical’s dry matter showed no significant differences between the two N fertilized treatments (Hoag and N-rec). Similar results were obtained by Sigurnjak et al. (2019) in a comparision of the fertilizing value of recovered ammonium sulphate and ammonium nitrate compared with mineral fertilization of lettuce and maize.
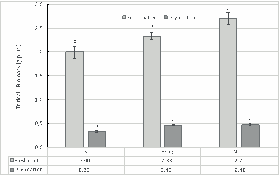
Figure 3 Biomass production of the triticale in the different treatments. Different letters above de columns indicate statistical differences (p <0.05) between the treatments by the Tukey test (capital letters between fresh matter, lower cases letters between dry matter).
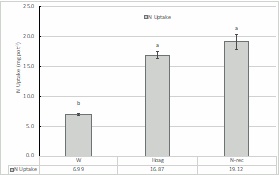
Figure 4 N uptake by the triticale in the different treatments. Different letters above de columns indicate statistical differences (p <0.05) between the treatments by the Tukey test.
Regarding the N uptake (Figure 4), the N-rec treatment showed the highest value although without significant diffrence from the Hoag. Despiste the experiment was conducted during a short period of time, the results pointed that at least the ammonium sulphate solution had a fertilizing effect similar to the mineral N fertilization.
CONCLUSIONS
The biomass production together with the N uptake of the triticale fertilized with N recovered from the digestate, was similar to the treatment fertilized with the Hoagland solution. So, the gas-permeable membrane technology showed to be a promising technology to recovered N from the effluents into a solution with crop fertilizing value.