Serviços Personalizados
Journal
Artigo
Indicadores
-
 Citado por SciELO
Citado por SciELO -
 Acessos
Acessos
Links relacionados
-
 Similares em
SciELO
Similares em
SciELO
Compartilhar
Portugaliae Electrochimica Acta
versão impressa ISSN 0872-1904
Port. Electrochim. Acta vol.32 no.3 Coimbra maio 2014
https://doi.org/10.4152/pea.201403183
Physicochemical Characterization and Corrosion Inhibition Potential of Ficus Benjamina (FB) Gum for Aluminum in 0.1 M H2SO4
Nnabuk O. Eddya,b,* , Paul O. Ameha , Anduang O. Odiongenyib
a Department of Chemistry, Ahmadu Bello University, Zaria Kaduna State, Nigeria
b Department of Chemistry, Akwa Ibom State University, Ikot Akpaeden, Mkpat Enin, Akwa Ibom State, Nigeria
Abstract
Examination of the physical (colour, odour, pH, solubility in various solvents) and chemical (GCMS and FTIR) characteristics of Ficus benjamina gum revealed that the gum is yellowish in colour, mildly acidic and ionic in nature. Major constituents of the gums were found to be sucrose and d-glucose, which constituted 60.92 % of their chemical constituents, while various carboxylic acids [(albietic acid (1.00%), hexadecanoic acid (4.41 %), 9-octadecanoic acid (1.00 %), octadecanoic acid (3.01 %), oleic acid (0.10 %), octadecanoic acid (9.12 %) and 6,13-pentacenequinone (20.43 %)] accounted for the remaining constituents. Functional groups identified in the gum were found to be those typical for other carbohydrates. From the knowledge of the chemical structures of compounds that constitute the gum, the corrosion inhibition potentials of the gum were ascertained and from weight loss analysis, the gum was found to be an active inhibitor against the corrosion of aluminum in solutions of tetraoxosulphate (VI) acid. The gum acted as an adsorption inhibitor that favours the mechanism of chemical adsorption and supported the Frumkin and Dubinin-Radushkevich adsorption models.
Keywords: Ficus benjamina gum, physicochemical characteristics and corrosion inhibition.
Introduction
Industrial revolution over the past centuries has provoked increase in the use of metals and their alloys for the fabrication of several installations. Some processes (such as acid wash, prickling and etching) within the oil, fertilizer, metallurgical and other industries expose metallic components to corrosion [1]. In view of this, several steps have been taken to protect metals against corrosion, but one of the best options involves the use of inhibitors [2,3]. Inhibitors are substances which, when present in minute quantity, are able to retard the corrosion of metal through the mechanism of adsorption [4].
Most of the suitable and documented corrosion inhibitors for metals are heterocyclic compounds [5-9] such as carbozones, quinolones, amines, amino acids, carbohydrates, polymers, plant extracts, chromates, etc. [10-13]. Of significant interest is the use of extract of plants and animals because they are natural, less toxic, biodegradable and easily accessible compared to those that are not ecofriendly [14].
The use of plant products as corrosion inhibitors is justified by the phytochemical components of the plant, some of which have chemical structures that meet the requirements needed for corrosion inhibition [15]. Exudates gums from plants have proven to be viable as corrosion inhibitors because they offer large adsorption sites, they are biodegradable, less toxic and can easily be gotten from gum bearing plants. Some of the gums that have been found to be good corrosion inhibitors include Khaya ivorensis gum exudate [16], Daniella Oliverri gum [17], Guar gum [18], Commiphora pedunculata gum [19], Ferula assa-foetida and Dorema ammoniacum gum exudates [20], Acacia seyal var. [14], Anogessius leocarpus gum [11], Ficus tricopoda [21], Ficus platyphylla gum [22] and Raphia hookeri gum [23]. Literature is however scanty on the use of Ficus benjamina gum as a corrosion inhibitor. Therefore, the present study is aimed at carrying out physicochemical, GCMS and FTIR analysis of FB gum and to investigate the corrosion inhibition potential of the gum.
Materials and methods
A sample of FB gum was tapped from the Ficus specie of the plant in Kanya Babba village, located in Babura Local Government arEa of Jigawa state, Nigeria. The crude sample of the gum was purified using the method described by Eddy et al., [17].
Physicochemical analysis
The pH of the gum was determined using a pre calibrated Oklon pH meter. The solubility of the gum was determined in cooled and hot distilled water, acetone and chloroform using the method reported by Carter (2005) [24].
FTIR analysis
FTIR analysis of the gum and that of the corrosion products (in the absence and presence of the gum) were carried out using a Scimadzu FTIR-8400S Fourier transform infra-red spectrophotometer. The sample was prepared in KBr and the analysis was carried out by scanning the sample through a wave number range of 400 to 4000 cm-1.
GC-MS analysis
GC-MS analysis was carried out on a GC Clarus 500 Perkin Elmer system comprising of a AOC-20i auto-sampler and gas chromatograph interfaced to a mass spectrometer (GC-MS) instrument employing the following conditions: column Elite-1 fused silica capillary column (30 x 0.25 mm ID x 1 μM df, composed of 100 % dimethylpoly diloxane), operating in electron impact mode at 70 eV; helium (99.99 %) was used as carrier gas at a constant flow of 1 mL/min and an injection volume of 0.5 μI was employed (split ratio of 10:1); injector temperature 250 °C; ion-source temperature of 280 °C. The oven temperature was programmed from 110 °C (isothermal for 2 min), with an increase of 10 °C/min, to 200 °C, then 5 °C/min to 280 °C, ending with a 9 min isothermal at 280 °C. Mass spectra were taken at 70 eV; a scan interval of 0.5 seconds and fragments from 40 to 450 Da. Total GC running time was 36 min. Interpretation on mass spectrum GC-MS was conducted using the database of National Institute Standard and Technology (NIST) Abuja, having more than 62 000 patterns. The spectrum of the unknown component was compared with the spectrum of the known components stored in the NIST library. The name, molecular weight and structure of the components of the test materials were ascertained. The concentrations of the identified compounds were determined through arEa and height normalization.
Corrosion studies
Aluminum alloy sheet of composition (wt. %, as determined by quantiometric method) Mn (1.28), Pb (0.064), Zn (0.006), Ti (0.029), Cu (0.81), Si (0.381), Fe (0.57), and Al (96.65%) was used for the study. The sheet was mechanically pressed cut into different coupons, each of dimension, 5x4x0.11 cm. Each coupon was degreased by washing with ethanol, cleaned with acetone and allowed to dry in the air before preservation in a desiccator. All reagents used for the study were analar grade and double distilled water was used for their preparation.
Weight loss experiments were carried out as reported elsewhere [10]. From weight loss measurements, inhibition efficiency, corrosion rate and degree of surface coverage were calculated using the following equations
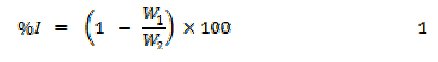


where W1 and W2 are the weight losses (g) for aluminum in the presence and absence of the inhibitor, Θ is the degree of surface coverage of the inhibitor, ΔW = W1 - W2, A is the arEa of the aluminum coupon (in cm2), t is the period of immersion (in hours) and ΔW is the weight loss of aluminum after time, t.
Results and discussion
Physicochemical parameters of Ficus benjamina gum
Physicochemical parameters of FB gum are presented in Table 1.
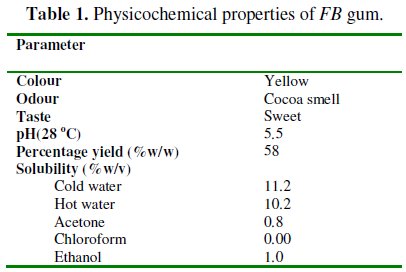
From the results obtained, it was found that FB gum is yellowish in colour (Fig. 1).

It has a sweet taste and exhibited an odour similar to that of cocoa. The gum is mildly acidic with a pH of 5.5. After purification, gum yield of 55 % was obtained. FB gum is ionic, soluble in cold and hot water. The solubility of the gum was found to decrease with increase in temperature. The gum is sparingly soluble in acetone and ethanol but insoluble in chloroform.
GC-MS and FTIR studies
The GCMS spectrum of FB gum is presented in Fig. 2 while Table 2 presents information deduced from the spectrum.
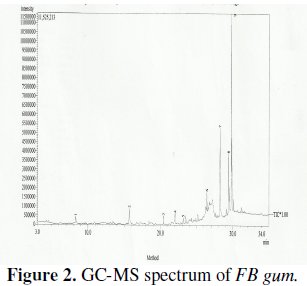
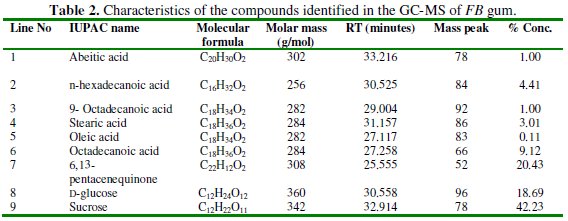
Names of identified compounds, chemical formula, molar mass and concentrations (gotten through arEa normalization) are also presented in Table 2, while the chemical structures of these compounds are also presented in Fig. 3.
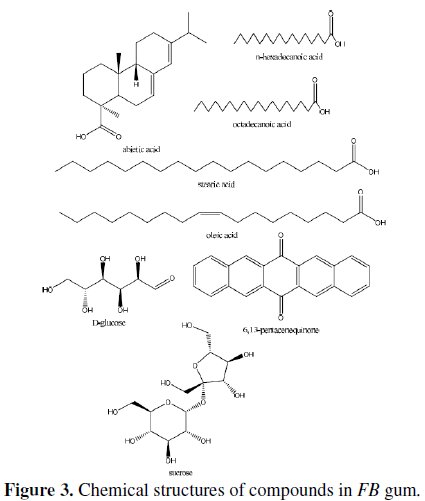
The spectrum revealed nine peaks, identified between the retention time range of 25.56 to 33.21 minutes. The identified compounds included, albietic acid (1.00%), hexadecanoic acid (4.41 %), 9-octadecanoic acid (1.00 %), octadecanoic acid (3.01 %), oleic acid (0.10 %), octadecanoic acid (9.12 %), 6,13-pentacenequinone (20.43 %), d glucose (18.69 %) and sucrose (42.23 %). From the above results, it can be seen that the dominant component of FB gum is carbohydrate (i.e., sucrose and d-glucose), constituting 60.92 % of the entire samples. Other samples were carboxylic acids indicating that, like other exudate gum, FB gum is rich in polysaccharides and carboxylic acids.
Fig. 4 presents the FTIR spectrum of FB gum.
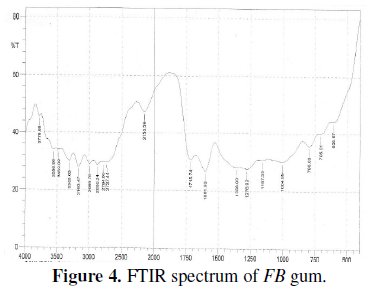
Peaks and frequencies of adsorption deduced from the spectrum as well as assigned bonds/functional groups are recorded in Table 3.
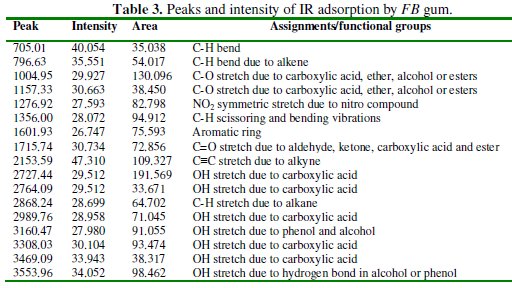
From the results obtained, it is evident that several stretches and vibrations, typical for polysaccharides are found in the FTIR spectrum of FB gum. These included OH stretches and vibrations due to carboxylic acids at 2727.44 and 2764.09, 2989.24, 3308.03 and 3469.09 cm-1, OH stretch due to alcohol or phenol was found at 3160.47. C-H stretch due to alkane at 2868.24 cm-1, C-H scissoring and bending vibrations at 1356.00 cm-1, C=O stretch due to aldehyde, ketone, ester and carboxylic acid, N-H stretch at 3308.03 and 3469.09, C-O vibration due to carboxylic acid and alcohol at 1004.95 cm-1. The presence of an aromatic ring at 1601 cm-1 was also observed.
Corrosion inhibition study
Effect of Ficus benjamina gum on the corrosion of Al
Fig. 5 shows the variation of weight loss with time for the corrosion of Al in solution of HCl containing various concentrations of FB gum at 303 and 333 K, respectively.
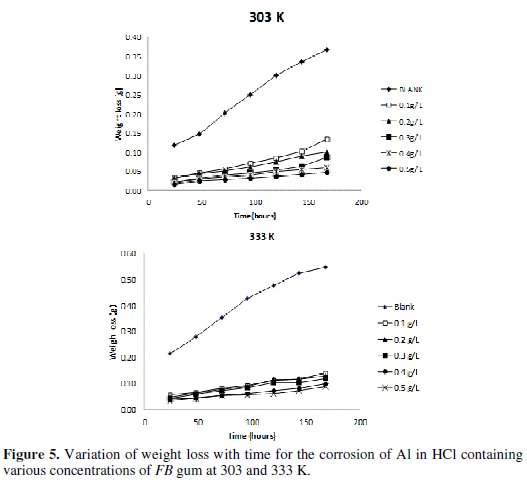
It is evident from the figures that the weight loss of mild steel increases with increase in the period of contact, but decreases with increase in the concentration of FB gum. These observations indicated that FB gum inhibited the corrosion of mild steel by reducing its rate of corrosion and that the inhibition efficiency of FB gum increases with increase in its concentration. Therefore, FB gum is an adsorption inhibitor, characterized by increase in inhibition efficiency with concentration [25].
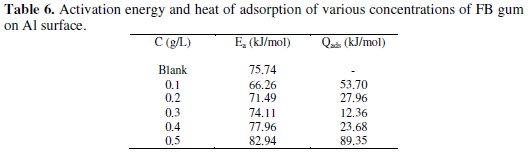
It was also found that the corrosion rate of Al in the presence of the inhibitor (Table 4) decreases with increase in temperature, while its inhibition efficiency increases with increase in temperature, indicating that the adsorption of FB gum on the surface of Al supported the mechanism of chemical adsorption.
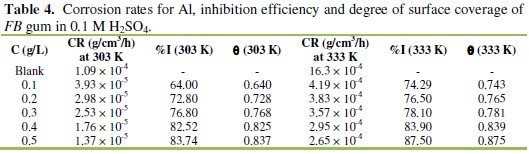
Chemisorption mechanism is characterized by increase in the extent of adsorption with temperature as oppose to physisorption mechanism, which compromises with decrease in the extent of adsorption with temperature [6].
Kinetic study
It has been found that most corrosion reactions are first order indicating that it follows a model, that can be represented as follows [17],
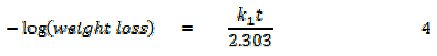
where t is the period of contact and k1 is the first order rate constant. Also, for a first order reaction, the half-life (t1/2) is related to the rate constant thus, t1/2 = 0.693/k1. Fig. 6 shows plots for the variation of âlog(weight loss) with time for the corrosion of Al in the absence and presence of FB gum as an inhibitor (at 303 and 333 K).
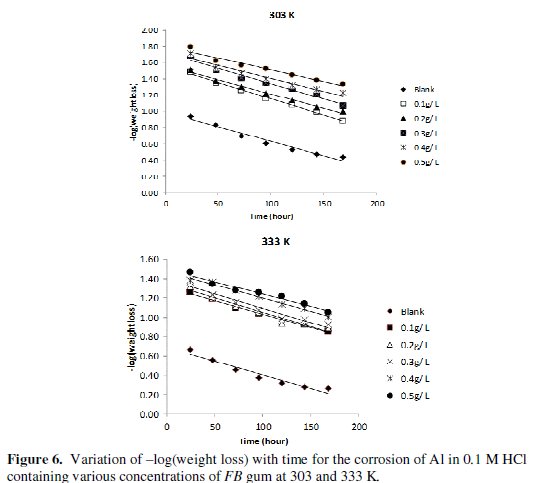
The plots revealed excellent correlation (R2 ≈ 1) indicating the application of eq. 4 to the corrosion of Al (in the absence and presence of FG gum). Values of k1 and t1/2 deduced from the slope of the plots are presented in Table 5.
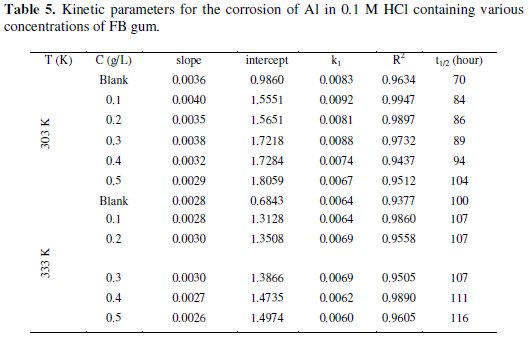
The half-lives for solutions containing various concentrations of FB gum are higher than that of the blank and were found to increase with increasing the concentration. Therefore, FG gum is a good inhibitor for the corrosion of Al.
Effect of temperature
Activation energies for the corrosion of aluminum in the absence and in the presence of FB gum were estimated using the logarithm form of the Arrhenius equation, which can be expressed as follows [12],
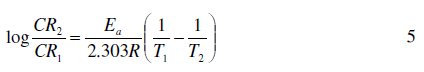
where CR1 and CR2 are the corrosion rates of mild steel at the temperatures T1 (303 K) and T2 (333 K), respectively, Ea is the activation energy, and R is the gas constant. Values of Ea calculated from eq. 5 are recorded in Table 5. From the results obtained, values of Ea ranged from 66.26 to 82.94 kJ/mol and are relatively comparable to the threshold value (80 kJ/mol) expected for the mechanism of chemical adsorption. Generally, lower values of Ea indicate a tendency towards physisorption mechanism, while higher values of Ea points towards chemisorption mechanism. The present results suggest that the adsorption of FG gum on the surface of Al must have first proceeded through the mechanism of physical adsorption and was succeeded by chemisorption mechanism.
Thermodynamic/adsorption considerations
The heat of adsorption of FB gum on Al surface was calculated using the following equation [22]
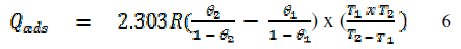
where R is the universal gas constant, θ2 and θ1 are the degree of surface coverage at the temperatures T1 (303 K) and T2 (333 K), respectively. Calculated values of Qads for various concentrations of FB gum are also presented in Table 5. The adsorption enthalpies are positive for all concentrations of FB gum indicating that the adsorption of FB gum on the surface of Al is endothermic. Adsorption isotherms are useful in studying the adsorption characteristics of corrosion inhibitors and were established by fitting the data obtained for degree of surface coverage into various adsorption models, including the Langmuir, Frumkin, Hill de-Boer, Parsons, Temkin, Flory-Huggin, Freundlich, Dhar-Flory- Huggin, kinetic/thermodynamic model of El-Awady et al. and Bockris Swinkels. The general form of an equation representing adsorption isotherms can be written as follows [26]
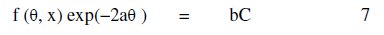
where f(Θ, x) is the configurational factor which depends upon the physical model and the assumptions underlying the derivation of the isotherm, Θ is the degree of surface coverage, C is the concentration of the inhibitor in the bulk electrolyte, x is the size factor ratio, 'a' is the molecular interaction parameter and 'b' is the equilibrium constant of the adsorption process. In this study, adsorption of FB gum was found to agree with the Frumkin adsorption model (eq. 8),
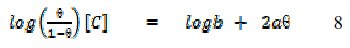
where Θ is the degree of surface coverage, C is the concentration of the adsorbate, b is the adsorption coefficient which represents the adsorptiondesorption equilibrium constant and 'a' is the interaction parameter. From eq. 8, a plot of log(Θ/(1-Θ))[C] versus Θ should be linear if Frumkin isotherm is obeyed.
Fig.7 shows the Frumkin isotherm for the adsorption of FB gum on the surface of aluminum.
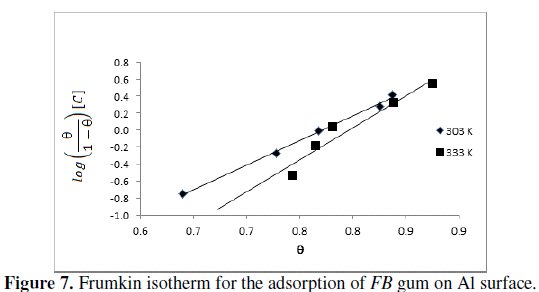
Adsorption parameters deduced from the plots are presented in Table 6.
The results revealed that the adsorption of FB gum on Al surface fitted the Frumkin model excellently (R2 = 0.9967 and 0.9381 at 303 and 333 K, respectively). The results also revealed that the interaction parameter (a = 2.8 and 3.8 at 303 and 333 K) is positive and increases with temperature, hence there is attraction between the adsorbed inhibitor's molecules and the extent of attraction increases with increase in temperature.
The equilibrium constant of adsorption, 'b', derived from the Frumkin model is related to the standard free energy of adsorption according to eq. 9 [26],
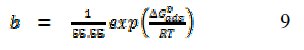
where ΔG0ads is the standard free energy of adsorption of FB gum on Al surface, 55.55 is the molar concentration of water in the acid solution, R is the gas constant, and T is the absolute temperature. In this study, calculated values of were -35.10 and -45.15 kJ/mol at 303 and 333 K. Although the free energy value at 303 K is slightly less than the threshold value (-40 kJ/mol), the calculated free energies nevertheless fail to support the mechanism of electron transfer from the inhibitor to a vacant d-orbital in the metal surface. The data also suggest that at lower temperature, the mechanism of physical adsorption was apparent while chemisorption dominated at higher temperature.
In order to confirm the mechanism of adsorption of FB gum on the surface of Al, the experimental data were also fitted into the Dubinin-Radushkevich isotherm model (D-RIM), expressed in eq. 10 [27]
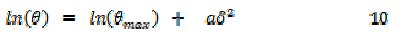
where Θmax is the maximum surface coverage and δ (Polany potential) can be correlated as:

The constant 'a' gives the mean adsorption energy, Eads, i.e., Eads = 1/(2a)0.5, R is the gas constant and T is the absolute temperature. The transfer energy (Eads) of 1 mol of adsorbate from infinity (bulk solution) to the surface of the adsorbent is an index for predicting the mechanism of adsorption of the inhibitor. When Eads is less than 8 kJ/mol, a physical adsorption mechanism is upheld and vice versa. The fitness of the data was excellent (R2 = 0.9616 and 0.8555 at 303 and 333 K) and calculated values of Eads were 10.00 and 10.50 kJ/mol at 303 and 333 K, respectively. This supports the mechanism of chemical adsorption as proposed earlier. Values of Θmax were calculated as 0.9148 and 0.9308 at 303 and 333 K, respectively, indicating that the maximum theoretical coverage for FB gum onto Al surface at 303 and 333 K corresponded to inhibition efficiencies of 91.48 and 93.08 %, respectively. The observed theoretical trend (i.e., increase in inhibition efficiency with temperature) shows slight deviation from the maximum inhibition efficiencies obtained from the experiments suggesting that these values may be attainable at higher concentrations (i.e., C > 0.5 g/L of FB gum) and not within the range of concentrations used in the present study. Nevertheless, the observed trend also supports the mechanism of chemical adsorption.
Mechanism of inhibition
The corrosion of Al in aqueous solution is a function of the concentration of anions in the system. HCl is a strong acid and can ionize to produce chloride ion, which can attack Al metal. The mechanism for the inhibition of the corrosion of aluminum in solution of HCl is similar to that proposed by Ford et al. [28-29] and is presented in eq. 12 to 15
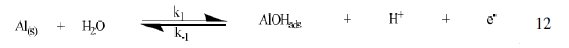
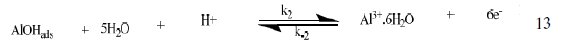
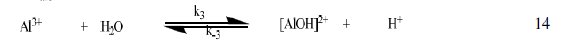
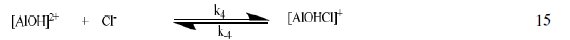
The controlling step in the metal dissolution is the complexation reaction between the hydrated cation and the anion (Eq. 15), which is feasible in the presence of chloride ions generated by ionization of HCl. Adsorption of the inhibitor on the surface of the metal will reduce the surface area available for anodic and cathodic reactions. According to Khaled [30], organic molecules inhibit corrosion by adsorption at the metal/solution interface and the adsorption depends on the molecule's chemical composition, the temperature and the electrochemical potential at the metal/solution interface. In case of aluminum surface, the solvent H2O molecules could also adsorb on the aluminum oxide surface forming hydroxylated sites, orhydroxide layers at the surface (Eq. 13 and 14), that impart a pH-dependent surface charge. The polar hydroxyl groups may cause the surface to attract and physically adsorb a single or several additional layers of polar water molecules. In acidic solution, the positively charged surface sites will electrostatically attract any anions present in solution, and replications. FB gum may be adsorbed on the aluminum oxide surface in four different ways [30]:
i. Electrostatic interaction between a negatively charged surface, which is provided by adsorbed chloride ion and the positively charged/protonated inhibitor,
ii. interaction of unshared electron pairs in the inhibitor's molecule with the aluminum surface,
iii. interaction of π-electron(s) with aluminum surface, iv. a combination of the (i-iii) types.
Efficient adsorption is the one that arises from interaction between π-electron(s) or hetero atom(s) in the FB gum molecule. Chemical adsorption seems to be the most important type of interaction between the Al2O3 surface and FB molecules. Here, the adsorbed species are in contact with the Al2O3 surface. In this process, a coordinated bond that involves the electron transfer from the inhibitor system towards the metallic surface is formed. The electron-transfer may be facilitated by lone pair of electrons in the inhibitor and the availability of π-electrons due to the presence of double bonds or aromatic rings in its structure. Moreover, there is a great possibility that adsorption may also take place via hydrogen bond formation between the XâH linkage (X may be hetero atom) in some of the constituents of FB gum and the oxygen atoms of the aluminum oxide/aluminum hydroxide surface species. This type of adsorption should be more prevalent for protonated X-atom(s), because the positive charge on the X-atom may be conductive to the formation of hydrogen bonds. Unprotonated X-atoms may also be adsorbed by direct chemisorption, as mentioned previously, or by hydrogen bonding to a surface oxidized species. The extent of adsorption by the respective modes depends on the nature of the metal surface. A good inhibitor must have strong affinity for the bare metal atoms. The requirement is different in case of aluminum; a compact passive oxide film is always present on the electrode surface, where hydrogen bond formation accounts for most of the inhibition action. An effective inhibitor is one that forms hydrogen bonds easily with the oxidized surface.
Conclusion
From the experimental results obtained in the present study, the following conclusion may be drawn
1. Ficus benjamina gum is yellowish odourless, mild acidic and ionic.
2. The chemical constituents of Ficus benjamina include albietic acid (1.00%), hexadecanoic acid (4.41 %), 9-octadecanoic acid (1.00 %), octadecanoic acid (3.01 %), oleic acid (0.10 %), octadecanoic acid (9.12 %), 6,13pentacenequinone (20.43 %), d glucose (18.69 %) and sucrose (42.23 %).
3. Ficus benjamina has been found to be a good adsorption inhibitor. Its inhibition proceeded via the mechanism of physical adsorption (the reasons are inhibition efficiency decrease with increasing temperature, Ea and ΔG0ads values were lower than the threshold values of 80 and -40 KJ/mol, and it was best described by Langmuir adsorption model.
4. Inhibition of mild steel by Ficus benjamina gum occurred through synergistic adsorption of the various components of the gums hence the formation of multiple adsorption layer is proposed.
References
1. Arora P, Kumar S, Sharma MK et al. E J Chem. 2007;4:450. [ Links ]
2. Ghasemi Z, Tizpar A. Appl Surf Sci. 2006;252:3667. [ Links ]
3. Elayyoubi S B, Hammouti S, Kertit HO et al. Rev Met Paris. 2004;2:153. [ Links ]
4. Rani B E A, Basu B B. Int J Corros. 2012;Article ID 380217. [ Links ]
5. Ita B I. Bull Electrochem. 2005;21:219323. [ Links ]
6. Ita B I. Proccurement Chem Soc Nig. 2004;10. [ Links ]
7. Ita B I. Bull Electrochem. 2004;20:363. [ Links ]
8. Ita B I, Offiong OE. Mater Chem Phys. 1997;51:203. [ Links ]
9. Fang J, Li J. J Mol Struct. 2002;593:179. [ Links ]
10. Eddy N O, Awe F E, Siaka A A, et al. Int J Electrochem Sci. 2011;6: 4316. [ Links ]
11. Eddy N O, Ameh P O, Gimba C E, et al. Int J Electrochem Sci. 2011;6:5815. [ Links ]
12. Ameh P O, Magaji L, Salihu T. Afr J Pure Appl Chem. 2012;6:100. [ Links ]
13. Umoren S A, Obot I B, Ebenso E E. E J Chem. 2008;5:355. [ Links ]
14. Buchweishaija J, Mhinzi G S. Electrochim Acta. 2008;26:257. [ Links ]
15. Oguzie E E, Enenebeaku C K, Akalezi C O, et al. J Colloid Interf Sci. 2010;349:283. [ Links ]
16. Ameh P O. Int J Modern Chem. 2012;2:28. [ Links ]
17. Eddy N O, Odiongenyi A O, Ameh P O, et al. Int J Electrochem Sci. 2012;7:7425. [ Links ]
18. Abdallah M. Port Electrochim Acta . 2004;22:161. [ Links ]
19. Ameh P O, Eddy N O. Res Chem Intermed. 2014;40:2641. [ Links ]
20. Behpour M, Ghoreishi S M, Khayatkashani M, et al. Corros Sci. 2011;53:2489. [ Links ]
21. Eddy N O, Ameh P O, Gwarzo M Y, et al. Port Electrochim Acta. 2013;31:79. [ Links ]
22. Eddy N O, Ameh P O, Gimba C E, et al. Int J Electrochem Sci. 2012;7:5677. [ Links ]
23. Umoren S A, Ebenso E E. Pigment and Resin Technology. 2008;37:173. [ Links ]
24. Carter S J. Tutorial Pharmacy: Solution. Great Britain:Pitman Press;2005. [ Links ]
25. Chetounani A, Hammouti B, Benkaddour M. Pigment Resin Technol. 2004;33:26. [ Links ]
26. Eddy N O. Port Electrochim Acta. 2009;27:579. [ Links ]
27. Khadom A A, Yaro A S, Musa A Y, et al. J Korean Chem Soc. 2012;56:406. [ Links ]
28. Ford F P, Burstein G T, Hoar T P. J Electrochem Soc. 1980;127:1325. [ Links ]
29. Foley R T T, Nguyen H. J Electrochem Soc. 1982;129:464. [ Links ]
30. Khaled K F. Corros Sci. 2010;52:2905. [ Links ]
*Corresponding author. E-mail address: nabukeddy@yahoo.com
Received 20 December 2013; accepted 10 May 2014














