Introduction
Iliac arteries aneurysms can develop several years after AAA repair. These are referred to as metachronous iliac aneurysms (MIA). There are a few cases described in the literature, and incidence rate after AAA open-repair is 10.2% in 10-year follow-up1-5. Even though in most cases MIAs do not grow substantially to warrant surgical repair, up to 9% of reported cases present with rupture2,6.
We report a rare case of bilateral MIAs (left CIA and right IIA) diagnosed 10-years after an aortobifemoral bypass. Aneurysms were successfully treated with bilateral endovascular exclusion. The left CIA aneurysm was treated by exclusion using a self-expandable stent graft (IIA to EIA endograft) in a “C” shaped manner (“banana” technique)7,8. The right IIA aneurysm was excluded after coil embolization and occlusion of the EIA. The patient gave consent for publication of the case report.
Case report
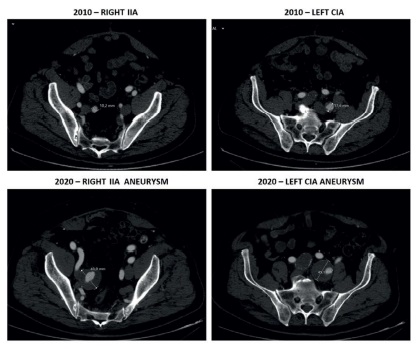
A 76-year-old man came for consultation after incidental finding of asymptomatic bilateral iliac artery aneurysms during the study of a renal cyst. Past medical history included obesity, smoking, hypertension, dyslipidemia, and ischemic heart disease. Ten years earlier, he had undergone emergent surgical repair of an 87-mm diameter ruptured AAA with a 39-mm diameter synchronous right CIA aneurysm. Intraoperatively the right CIA had been isolated and ligated distally to the right CIA aneurysm. The left CIA had been oversewn at the ostium after the sac was opened because of its tortuous path that complicated isolation. Due to the emergent character of the procedure an aortobifemoral bypass had been performed and flow was preserved to the iliac arteries by retrograde perfusion. At that time the left CIA was 18-mm in diameter and the right IIA was 10-mm in diameter (Figure 1).
A computed tomography angiography (CTA) scan was performed and confirmed aortobifemoral graft patency with the presence of a 43-mm diameter right IIA aneurysm and a 45-mm diameter left CIA aneurysm (Figure 1). Both EIAs and left IIA were normal in diameter. Common and superficial femoral arteries were bilaterally patent. Because of the presence of a hostile abdomen, the deep location of the iliac aneurysms and the patient increased surgical risk, we decided to perform an endovascular exclusion of both iliac aneurysms. We opted to occlude the right IIA aneurysm with coil embolization and to exclude the left CIA aneurysm with an IIA to EIA self-expanding endograft, positioned in a “C” shaped manner (“banana” technique)7-9.
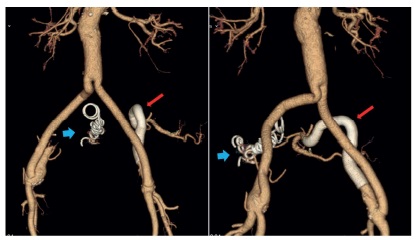
The procedure was performed under general anesthesia because of the expected length of surgery and the need for surgical approach of the superficial femoral artery (SFA). We considered surgical approach of the SFA to be safer due to the need to use large sheaths and because CFA percutaneous access was limited owing to previous aortobifemoral bypass. Retrograde access was obtained after bilateral SFA approach and insertion of 6F sheaths. The left IIA was cannulated using a 0.035-in hydrophilic guidewire and a cobra catheter. A superstiff guidewire was then positioned in the IIA and the left access was exchanged to a 10F sheath. After administration of 2 500 units of heparin a 10×100mm self-expanding endograft (Viabahn, W. L. Gore, Flagstaff, Ariz) was delivered from the distal IIA to the EIA to exclude the left CIA aneurysm. The implant was distally completed by inserting a 13×100mm self-expanding endograft with 5-cm overlap. We used endografts with different diameters because of the difference in sizes between the distal IIA (diameter of 9mm) and the EIA (diameter of 11mm). Final angiogram confirmed complete exclusion of the left CIA aneurysm with preservation of the internal iliac flow through the “C” shaped endograft (Figure 2).
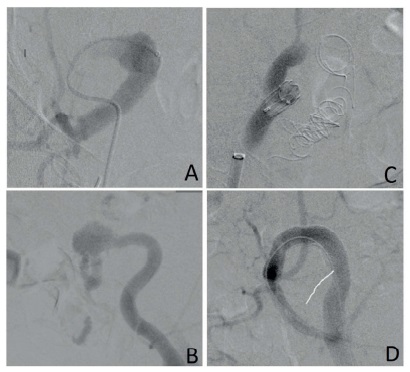
Figure 2 Pre-procedural angiograms showing patent right IIA (A) and left CIA (B). Post-procedural angiograms showing total occlusion of right IIA aneurysm after coil embolization and occlusion of the EIA (C), and total exclusion of the CIA aneurysm with an IIA to EIA endograft in a “C” shaped manner (D).
The right IIA aneurysm was then cannulated in the same fashion and a superstiff guidewire was used to introduce a 65-mm 6F sheath in the distal portion of the aneurysm. To distally exclude the IIA aneurysm, embolization of both anterior and posterior IIA branches was performed using 8-mm and 10-mm coils (Nester Embolization Coils, Cook, Inc., Bloomington, IN). The 6F sheath was then retracted and embolization of the aneurysm sac was performed with two 20-mm coils. Occlusion of the proximal EIA was achieved with delivery of a 14-mm occluder in a 17.5F sheath (Talent Occluder, Medtronic, Minneapolis, Minn). The final angiogram confirmed complete occlusion of the right IIA aneurysm (Figure 2). Total procedure time was 2 hours and 54 minutes.
There were no adverse events in the postoperative period and the patient was discharged after two days in good general condition. Dual antiplatelet therapy with clopidogrel 75 mg and acetylsalicylic acid (ASA) 100 mg once daily was prescribed for a month with subsequent ASA long-term therapy. CTA scan two months after surgery showed complete success of the procedure with total exclusion of the left CIA aneurysm without endoleaks, good positioning and patency of the stent grafts and with normal filling of the left IIA artery, as well as complete occlusion of the right IIA aneurysm and EIA (Figure 3). At 12-months follow-up the patient is asymptomatic, with no complications associated to the procedure or reinterventions needed.
Discussion
The development MIAs in iliac arteries excluded from high-pressure direct flow from the aorta is very uncommon and there are only a small number of cases described in the literature1,3-5,10,11. When aortobifemoral bypass grafts are performed for AAA open-repair, iliac vessels perfusion is maintained only by retrograde flow from the femoral arteries. There is little evidence available concerning the natural history of iliac circulation with retrograde perfusion after AAA open-repair. In 1998, Hill et al12 presented a series of 32 patients with AAA treated by aortobifemoral bypass with a mean follow-up time of 39.3 months and reported that iliac arteries remained stable in size or thrombosed in all patients. This study included patients with pre-existing iliac artery dilation or atherosclerotic disease, emphasizing the low risk of aneurysmal degeneration with retrograde perfusion in the short-term even in high-risk patients. The fate of iliac circulation after aortobifemoral bypass in the long-term is still undefined. Because MIAs reported in the literature required treatment between 6 to 13 years after aortobifemoral AAA open-repair, long-term follow-up is needed to better understand the risk of aneurysmal degeneration and rupture1,3-5,9,11.
Theoretically, by preserving direct anterograde flow to the iliac vessels, aortic tubes render a greater risk of progression of iliac aneurysmal disease when compared to aortobifemoral grafts. There appears to be no evidence in the literature to corroborate this belief. The fate of iliac circulation after aorto-aortic graft repair of AAA, with preservation of anterograde flow, has been reported in several clinical series2,13-15. Lavee et al14 (1988) followed 23 patients for 5 years after AAA tube repair and reported no significant aneurysmal degeneration of iliac vessels. Ballotta et al2 (2008) prospectively followed 201 patients for a mean of 7.1 years and reported low rates iliac arteries growth, with development of iliac aneurysms above 25mm only in 3 patients, none of which required surgical repair. Other authors reported similar results, concluding that most CIAs do not expand after tube graft insertion for AAA open-repair, and even when they do the degree of dilation is minimal13,15,16.
MIAs are usually detected during follow-up or surveillance imaging of patients previously submitted to AAA open-repair. Most patients are asymptomatic (43%), but up to 9% may present with rupture6. Most frequent symptoms are abdominal pain (35%), limb ischemia (9%) and hydronephrosis (4%)6. The management of MIAs after AAA open-repair may involve surgical exclusion with bypass graft, ligation or endoaneurysmorrhaphy. Surgical treatment of iliac aneurysms can be challenging because of the risk of major complications such as ureteral or venous injury, damage to adjacent viscera or nerves, acute limb ischemia and death17. In patients with previous intra-abdominal surgery, the risks associated with open reintervention are even higher. Iliac aneurysms open-repair is associated with high early-morbidity rates (43%) when compared to endovascular treatment (8%)17. Series report mortality rates up to 10%, higher than those reported for AAA open-repair18,19.
In recent years, endovascular treatment of iliac aneurysms with embolization, iliac branch endograft placement or IIA to EIA endografting proved to be a safe, simple and effective solution, associated with low morbidity and mortality rates17,18. However, even these techniques are not free from complications such as graft thrombosis, endoleaks, and conversion to open surgery. The introduction of minimally invasive endovascular options in the treatment of MIAs has unlocked less aggressive ways of treatment. However, maintaining the patency of pelvic circulation must be considered when choosing the best approach. Sacrifice of unilateral or bilateral IIA perfusion is not benign and leads to pelvic ischemic complications in a significant proportion of patients. The overall incidence of buttock claudication was 28% in patients with unilateral occlusion of IIA, and 42% in patients with bilateral IIA embolization. Younger and physically active patients were at higher risk of developing these symptons20. In the case presented we opted to embolize the right IIA aneurysm because it ended at the bifurcation of the IIA into anterior and posterior divisions and did not present an adequate sealing length, making it technically challenging for endovascular repair. Maintaining patency of the left IIA was therefore fundamental to avoid severe complications such as gluteal/perineal skin necrosis, spinal cord ischemia and intestinal ischemia21.
The use of an IIA to EIA endograft in the treatment of iliac artery aneurysms has been previously described in the literature. In 1999, Hoffer et al8 first described its use in the treatment of bilateral CIA aneurysms combined with an aortouni-iliac stent graft, right IIA coil embolization and a right to-left femoral-femoral bypass graft. Since then, different authors have reported its effectiveness in the management of MIAs originating several years after aortobifemoral grafting for AAA open-repair1,9-11. In 2011, Mosquera Arochena et al7 described the procedure in the treatment of bilateral CIA aneurysms and named it the “banana” technique. Procedural success of this technique depends on choosing an appropriate stent. The advantage of using this endograft (Viabahn, W. L. Gore, Flagstaff, Ariz) is that it is extremely flexible, minimizing the risk of occlusion because of kinking in the tortuous iliac arteries.
The case described highlights the risk of aneurysmal degeneration of iliac vessel after aortobifemoral graft for AAA open-repair and demonstrates that retrograde perfusion of iliac vessels can lead to MIAs formation in the long-term. Longer follow-up and surveillance imaging of patients after AAA open-repair with aortobifemoral grafts must be contemplated, especially because most MIAs are asymptomatic and presentation might occur only at rupture.
Conclusion
Although this case describes a rare occurrence, it highlights that aneurysmal degeneration of iliac arteries can be generated by retrograde blood flow after treatment of AAA by aortobifemoral bypass. Endovascular embolization of the right IIA aneurysm, combined with a “C” shaped left IIA to EIA stent-endograft to exclude the left CIA aneurysm was effective in treating both aneurysms while preserving flow to the left IIA.