Introduction
Acute mesenteric ischemia (AMI) constitutes a life-threatening emergency, presenting a persistent diagnostic challenge despite advancements in diagnostic techniques. Estimates suggest it may account for approximately 1% of all cases of acute abdomen and may reach 10% in patients over 70 years old.1,2 Arterial thromboembolic occlusion emerges as the predominant cause, detected in up to 68% of cases, while venous thrombosis and non-occlusive etiologies represent 28% and 7%, respectively.3,4 Nevertheless, the true incidence likely remains underreported due to undiagnosed cases. As early as 1926, Cokkinis lamented the seemingly insurmountable challenges posed by this condition, stating “the diagnosis is impossible, the prognosis hopeless, and the treatment useless”.5 Despite a century of medical progress, AMI remains a source of concern, particularly due to poor outcomes, even after revascularization. Notably, despite advancements in patient management strategies, mortality rates have shown limited improvement over the past decades,6 highlighting an enduring gap in our understanding of the pathology and its prognostic indicators. This study aimed to evaluate real-world outcomes among patients admitted for acute mesenteric arterial occlusive ischemia, focusing on cases referred to vascular surgery where mesenteric revascularization was attempted.
Methods
A retrospective single-center analysis was conducted, comprising all patients who underwent emergent surgery for occlusive arterial AMI between January 2020 and February 2024. Demographic, clinical, and procedural data were extracted from patient records. The primary outcome assessed was the 30-day survival rate. Secondary analysis included the evaluation of potential laboratory predictors of in-hospital mortality, which were rapidly obtained at admission through arterial blood gas and venous analysis. Previously described parameters, including acid-base imbalances (pH and lactate), renal function (creatinine), and neutrophil-to-lymphocyte ratio (NLR), were included in the analysis.7-11
Although urea and the urea-to-creatinine ratio have not been widely studied in this context, they are promising indicators for evaluating critically ill patients and were therefore incorporated.12 The inclusion of bicarbonate as a variable, both independently and in combination with lactate variability - HCO3-to-lactate ratio - was based on our empirical observation that patients with greater clinical severity exhibited not only elevated lactate levels, a well-established marker in the literature, but also significant bicarbonate depletion, a key buffer in acid-base disturbances. Values from the last preoperative samples were used to analyze all parameters. The interval between the onset of acute symptoms and operation was also investigated as a prognostic factor. Statistical analyses were conducted using IBM's SPSS Statistics version 25, with a significance level set at 0.05. Categorical variables were summarized as frequencies and percentages. Normal distribution of continuous variables was assessed using the Shapiro-Wilk test. Continuous variables with normal distribution were presented as means and standard deviations and compared using t-tests; variables with non-normal distribution were expressed as medians and interquartile ranges and compared through the Mann-Whitney test. The 30-day survival rate was calculated using the Kaplan-Meier method. Differences in survival rates among different surgical approaches (open, endovascular, and hybrid) and between embolic and thrombotic etiologies were assessed using the Mantel-Cox log-rank test. Potential predictors of 30-day mortality and their impact on prognosis were analyzed using the Cox proportional hazard model (stepwise backwards multivariable analysis).
Receiver operating characteristic (ROC) curve analysis was performed to assess the discriminative ability of significant variables in predicting outcomes. DeLong's test was employed to compare the Area Under the Curve (AUC) values, with the optimal threshold determined based on the Youden index.
Results
Thirty patients, with a mean age of 80 years, underwent emergent surgery for occlusive arterial AMI. Males were predominantly affected, with a ratio of females to males of 1:1.3. In most patients, the index event was the first presentation of mesenteric ischemia, with only six reporting previous symptoms suggestive of intestinal angina. The median time of acute symptom evolution was 48 hours [12 - 96], and the mean time from diagnosis to surgery was 1.8 hours [0.5h - 6]. An embolic cardiac source was identified in 14 (47%) patients. The majority underwent open surgical thromboembolectomy (n=17), with retrograde open stenting of the superior mesenteric artery (ROMS) in seven of these patients. Segmental enterectomy was performed in five patients during the index operation, with second-look laparostomy at 24 hours performed in 10 patients, three requiring enterectomy at this time-point, Table 1. The overall 30-day survival rate was 33%, Figure 1. When comparing thrombotic and embolic etiologies, there was a non-significant trend to higher 30-day survival rates (44% vs. 21%, p=0.266) in the thrombotic group, Figure 2. Comparing different revascularization strategies (endovascular, hybrid, or open), the endovascular group exhibited a higher 30-day survival rate compared to hybrid and open approaches, though without statistical significance (30-day survival rates of 50%, 25%, and 10%, respectively; p=0.482), Figure 3.
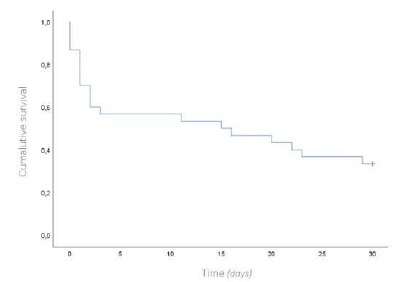
Figure 1 Kaplan-Meier curve for overall survival at 30-days for patients with acute mesenteric ischemia
Analysis of preoperative variables revealed that the HCO3-to-lactate ratio significantly impacted 30-day survival, with a 15% increase in mortality for each unit decrease in the ratio (Hazard Ratio 0.846, 95% confidence interval 0.760-0.943, p=0.002). Additionally, the NLR showed a significant relation with 30-day survival, with a 4% hazard increase in mortality for each unit increase in NLR (Hazard Ratio =1.044, 95% confidence interval 1.012-1.077, p=0.006). Creatinine, urea, and time of symptom evolution showed no significant relation with the outcome, Tables 2 and 3.
Table 1 Demographics, presentation, etiology, and operative details of patients with acute mesenteric ischemia included in this study.
| Male sex, n(%) | 17 (57) |
| Age, mean(SD) | 79.6 (9.7) |
| Presentation | |
| Acute, n(%) | 24 (80) |
| Acute on chronic, n(%) | 6 (20) |
| Time to diagnosis (h), median (IQR) | 48 (48) |
| Time from diagnosis to OR (h), mean (SD) | 1.8 (1.6) |
| Etiology | |
| Embolic, n(%) | 14 (47) |
| Thrombotic, n(%) | 16 (53) |
| Atherosclerotic, n(%) | 15 (94) |
| Isolated SMA dissection, n(%) | 1 (6) |
| Operative strategy | |
| Thromboembolectomy, n(%) | 10 (33) |
| Thromboembolectomy + ROMS, n(%) | 7 (23) |
| Thromboaspiration (Penumbra®), n(%) | 2 (6) |
| Pharmacomechanical thrombectomy (Angiojet®) + stent, n(%) | 1 (3) |
| Angioplasty + stent, n(%) | 6 (20) |
| Exploration without revascularization, n(%) | 4 (13) |
| Enterectomy, n(%) | 5 (17) |
| Second-look exploration at 24h, n(%) | 10 (33) |
| Enterectomy at second-look, n(%) | 3 (30) |
h - hours; OR - operating room; SD - standard deviation; SMA - superior mesenteric artery; ROMS - retrograde open mesenteric stenting
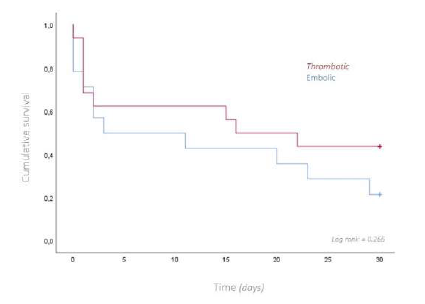
Figure 2 Kaplan-Meier curve for overall survival at 30-days, comparing patients with thrombotic and embolic etiology for acute mesenteric ischemia.
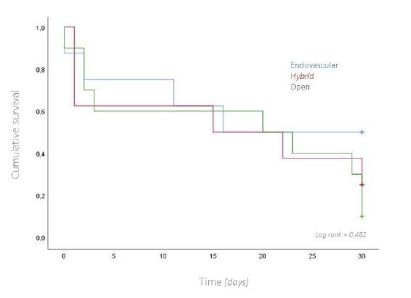
Figure 3 Kaplan-Meier curve for overall survival at 30-days, comparing patients with different operative strategies (endovascular, hybrid or open) for acute mesenteric ischemia.
Table 2 Predictors of 30-day mortality for patients with acute mesenteric ischemia, included in the study (univariable analysis)
| Dead at 30 days | Alive at 30 days | p | |
|---|---|---|---|
| pH, mean ± SD | 7.34 ± 0.02 | 7.41 ± 0.02 | 0.340 |
| Lactate, mean ± SD | 6.15 ± 1.23 | 1.66 ± 0.33 | 0.004 |
| HCO3, mean ± SD | 19.57 ± 1.04 | 24.12 ± 1.19 | 0.350 |
| HCO3:Lactate, mean ± SD | 6.50 ± 1.18 | 21.40 ± 4.98 | 0.009 |
| Neutrophiles, mean ± SD | 14670 ± 1342 | 10251 ± 1373 | 0.417 |
| Lymphocytes, mean ± SD | 847 ± 132 | 1303 ± 208 | 0.551 |
| NLR, mean ± SD | 24.67 ± 3.62 | 9.60 ± 1.76 | 0.016 |
| Creatinine, mean ± SD | 1.82 ± 0.2 | 1.08 ± 0.14 | 0.099 |
| Urea, mean ± SD | 79.67 ± 7.62 | 66.44 ± 9.62 | 0.391 |
| Urea:creatinine, mean ± SD | 47.11 ± 4.39 | 57.7 ± 10.86 | 0.354 |
| Time to diagnosis, median (IQR) | 72 (48) | 48 (48) | 0.846 |
IQR - interquartile range; NLR - neutrophile-to-lymphocyte ratio; SD - standard deviation.
Table 3 Predictors of 30-day mortality for patients with acute mesenteric ischemia, included in the study (multivariable analysis)
| HR | p | CI | HR | p | CI | |
|---|---|---|---|---|---|---|
| pH | 24.69 | 0.421 | 0.010 - 61365.77 | |||
| Lactate | 0.99 | 0.895 | 0.883 - 1.173 | |||
| HCO3 | 0.87 | 0.125 | 0.721 - 1.041 | |||
| HCO3:lactate | 0.87 | 0.028 | 0.776 - 0.985 | 0.846 | 0.002 | 0.760 - 0.943 |
| Neutrophiles | 1.091 | 0.182 | 0.960 - 1.240 | |||
| Lymphocytes | 0.684 | 0.628 | 0.147 - 3.185 | |||
| NLR | 1.02 | 0.537 | 0.958 - 1.085 | 1.044 | 0.006 | 1.012 - 1.077 |
| Creatinine | 0.906 | 0.923 | 0.122 - 6.758 | |||
| Urea | 1.01 | 0.689 | 0.962 - 1.060 | |||
| Urea: Creatinine | 0.973 | 0.404 | 0.911 - 1.038 | |||
| Time to diagnosis | 1.003 | 0.777 | 0.985 - 1.021 |
CI - confidence interval; HR - hazard ratio; NLR - neutrophile-to-lymphocyte ratio
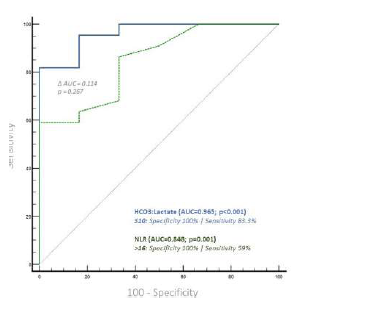
ROC analysis determined a HCO3-to-lactate ratio ≤ 10 as the optimal cutoff for predicting 30-day mortality, exhibiting a specificity of 100% and a sensitivity of 83% (AUC=0.965; CI 0.826-0.999; p<0.001).
For NLR, the optimal cutoff was > 16, with a specificity of 100% and a sensitivity of 59% (AUC 0.848; CI 0.663-0.955; p<0.001). Pairwise comparison of the Areas Under the Curves (AUCs) showed a difference of 0.114, which was not statistically significant (p=0.2647), Figure 4.
Discussion
The overall mortality of acute mesenteric ischemia remains alarmingly high, as evidenced by the 30-day survival rate of 33% observed in patients with occlusive arterial AMI referred for revascularization in this study. These results fall short of those reported in the literature,13,14 indicating the persistent challenge posed by this condition and the inadequacy of current diagnostic and management strategies. Despite not achieving statistical significance in the multivariable Cox regression analysis, it is noteworthy that the majority of patients experienced a significant delay in diagnosis and referral to the vascular team following the onset of acute symptoms, with a median time of symptom evolution of 48 hours - a marked deviation from the reported average delay of 8 hours, with a reported increase in mortality rates to 80-100% with delays of more than 24 hours, the authors assume that this lack of significance may be justified by the small sample size and by the globally prolonged time of evolution, which precludes the evaluation of the true benefit of a timely revascularization.15,16
When comparing subgroups, patients with thrombotic etiology and those treated with endovascular approaches had higher survival rates, although without statistical significance, possibly due to the small sample size. We hypothesize that the higher survival trend in the thrombotic group is due to the inclusion of patients with acute-on-chronic presentations. These patients often present with a less severe clinical picture, owing to established collateral circulation, which allows for less invasive endovascular management, which is also associated with lower mortality in this subgroup.14 It remains unclear whether patients treated with endovascular techniques have better survival due to the treatment itself or because it is offered to patients with a better baseline prognosis.
It has been shown that a timely revascularization significantly improves survival of these patients. Yet, a proper diagnosis and timely revascularization are still a challenge, prompting questions about optimizing the pathology's recognition and defining the optimal window for intervention.13 Various prognostic factors have been proposed with a recent meta-analysis, including 51 studies and 10 394 patients, showing that advanced age, patient’s dependency, delay to surgery, chronic kidney disease, diabetes, arrhythmia, cardiac failure, hemodynamic instability, extensive bowel involvement and higher levels of creatinine and lactates correlated with mortality.7 Given the limited sample size, this study focused on parameters rapidly obtained during the patient’s evaluation. Lactate levels have been widely studied as prognostic markers. Still, their utility in predicting outcomes remains contentious, particularly given their tendency to stay within normal ranges until late stages of the disease, and data showed that the value of serial lactate and pH is limited in predicting the extent of intestinal ischemia.8,9 However, it is also crucial to recognize that, despite not being sensitive enough to guide diagnosis, these patients experience significant metabolic imbalances, which may significantly impact the prognosis.
This study failed to show that the lactates or the pH alone related with 30-day survival; however, when analyzing the combined variation of the lactate and the HCO3 a statistically significant result was found, with a HCO3-to-lactate ratio ≤ 10 was showing a 100% specificity and an 83% sensitivity for 30-day mortality and independent of the creatinine levels. The authors theorize that the lactate-to-HCO3 ratio may serve as an indicator not only of the severity of ischemia and tissue hypoperfusion but also reflects the impairment of acidemia buffering and correction mechanisms to compensate for metabolic dysfunctions, which may be compromised, particularly in critically ill patients, and may dictate a status of irreversibility.17 Consequently, encompassing both factors, this composite index proves highly predictive of mortality. A recently published study, applying a similar rationale, found that the inverted lactate-to-bicarbonate ratio had greater predictive power for 28-day in-hospital mortality in children with dengue septic shock compared to lactate alone (Odds Ratio 8.66, p < 0.01 vs. 1.35, p < 0.001, respectively), reinforcing the prognostic significance of this index in critically ill patients.18
The neutrophil-to-lymphocyte ratio has been proposed as a prognostic tool in other vascular territories,19-22 and in patients with acute mesenteric ischemia.11 In this study, we corroborate this hypothesis, with an NLR ≥ 16 showing a 100% specificity for 30-day mortality.
If the time to diagnosis and referral for revascularization, far beyond the ideal 12 hours before peritonitis and septic shock has been established, is still a limiting step for adequate management, the challenge remains after diagnosis is made in determining whether the window for revascularization may have elapsed.15 Also, in the era of endovascular treatment, the need for first or second-look laparotomy and the post-operative level of care is not always clear. Both these ratios are rapidly available during the perioperative evaluation and may guide decision-making. Their exceptionally high specificity translates the profound impact of metabolic imbalances and inflammatory response on the prognosis of these patients. It may indicate the irreversibility of the condition and predict the futility or need for additional interventions.
The urea-creatinine ratio is a marker of pre-renal acute kidney injury associated with hypoperfusion states and higher mortality. It has been proposed as a prognostic marker associated with catabolism in critically ill patients.12,23 This ratio failed to show an impact on the 30-day survival in this study. However, it may have a potential interest during the follow-up, when evaluating the long-term survival, considering the extended hospitalization periods with muscle wastage and impaired nutritional status associated with extensive bowel resections, contributing to high-catabolic states, which are frequent among the patients surviving the acute stage.
The primary limitations of this study include its retrospective, single-center design and small sample size, which restricted the ability to perform comprehensive subgroup analyses and evaluate other critical care measures beyond revascularization procedures. Furthermore, the absence of a standardized postoperative protocol for serially measuring these markers prevented an assessment of how the implemented interventions influenced patient outcomes. As a pilot study, these findings should be considered preliminary. Future research with a larger, multi-center cohort and standardized protocols is essential to address these limitations and validate the study’s premises.
Conclusion
The short-term survival rate reflects the impact of delayed diagnosis and highlight the poor prognosis associated with acute mesenteric ischemia, underscoring the suboptimal outcomes in this patient population. Preliminary data on the lactate-to-HCO3 and NLR, which demonstrate high specificity for predicting 30-day mortality, suggest their potential as valuable prognostic markers. These findings offer promising insights into risk stratification in this critically ill population and hold potential for translation into clinical practice to guide decision-making.