Introduction
Vascular reconstruction using prosthetic vascular grafts has revolutionized the management of aortic diseases in the twentieth century, leading to a significant improvement in the quality of life of patients with vascular disease.1 Nevertheless, vascular graft infections (VGI), particularly in an intracavitary setting, persist as a rare but devastating complication of aortic surgery. This complication may affect both patients that underwent open aortic repair and endovascular aneurysm repair.2,3 Despite aggressive antimicrobial therapy and surgical treatment, the mortality and morbidity rates remain high.3 Reported incidence of intracavitary (i.e. intra-thoracic or intra-abdominal, such as aorto-femoral, aorto-iliac or ilio-femoral) aortic graft infections varies between 0.2% and 5%.4-6 Nevertheless, there are 2 characteristics that make its true incidence hard to assess: it varies according to anatomical site and emergency setting;4 and clinical signs of infection can appear months or years after index surgery.4,7
As there is a lack of consensus and standardized evidence on aortic graft infections, we aim to summarize available data regarding key aspects of aortic graft infections, such as pathogenesis, risk factors, microbiology, diagnosis and treatment strategies.
Methods
We conducted a literature search in Pubmed using the following search terms, either isolated or combined: “vascular graft infection”, “prosthetic infection”, “aortoenteric fistula”, “aortic repair”, “aortic surgery”. This narrative review follows the more recent publishing recommendations reported on the Scale for the quality assessment of narrative review articles (SANRA).8
Results
Pathogenesis and risk factors for infection
The pathogenesis of VGI is not fully understood and is likely multifactorial.
There are 2 peaks in onset of VGI after graft implantation. The first and most common occurs in the early postoperative period and is most likely related to intraoperative bacterial contamination of prosthetic graft or due to spread of infection from a contiguous site, namely following surgical wound infection. The second peak occurs at a later time and can happen due to extension from an intracavitary abscess, bacterial colonization of a thrombus, direct inoculation of infection during other surgical procedures (i.e. percutaneous drainage of a fluid collection or an abscess), new bacteremia or reactivation of a dormant graft infection.4 Aortoenteric fistulas can also be a source of infection in over 30% of intra-abdominal VGI, most often as a result of erosion of a graft into the duodenum and less frequently into the colon.4
VGI are thought to happen most often due to either contamination at the index surgery, hematogenous infection or spread from a contiguous site. The risk of hematogenous VGI is highest in the first 2 months and decreases over time thanks to partial endothelialization of the graft.4 Nevertheless, as the pseudo-intimal lining can take up to 1 year after implantation to develop, the vascular graft remains susceptible to secondary infection due to bacteremia.9 Contamination during surgery can occur due to the use of a breach in graft or procedure sterility, contamination from the patient’s contiguous skin or exposure to visceral contents (namely in concomitant biliary, bowel and urinary procedures), or deposition of airborne particles into the surgical bed. This is particularly true in an emergency setting, with a more frequent loss of asepsis. In an observational study from Shiraev and colleagues, emergency surgeries carried a significantly higher risk for further graft infection.10 The most common cause of graft infection is contamination from the skin of the groin, since wound healing complications in this area are common, and in obese patients the surgical wound lies within moist skin folds.3 Moreover, patients that need graft revision due to failed vascular reconstruction frequently harbor bacteria within scar tissue and lymphoceles and on the surfaces of previously implanted prosthetic vascular grafts and suture material.9
Even though infection has no direct significant adverse effect upon the graft itself, the infection will inevitably spread to the host tissue, resulting in inflammation and disruption of the graft - artery anastomosis, leading to the development of a false aneurysm, graft - enteric fistula, hemorrhage, which can cause limb loss or death.9 Additionally, the resulting bloodstream infection, secondary to a graft source of infection can result in a metastatic spread to other organs, sepsis and multiorgan failure, while being a difficult to treat sanctuary, that often requires prolonged antimicrobial therapy.4
In summary, risk factors for intracavitary graft infections may be categorized into patient-related comorbidities (such as diabetes, smoking, advanced age and chronic kidney disease), as well as procedure-related factors (location and type of graft, postoperative hyperglycemia, bacteremia, emergency setting).2,5 On this subject, Vogel and colleagues analyzed risk factors in over 10000 patients submitted to abdominal aortic aneurysm repair. In their cohort, bacteremia reported in the index admission for repair was significantly associated with aortic graft infection (odds ratio [OR], 4.2; 95% confidence interval [CI], 1.5-11.8).2 Local infection, such as surgical site infection or groin infections, as well as dehiscence, represent potential bacterial entry points.4,11 Regarding hyperglycemia, a retrospective cohort study performed by fellow surgeons in Amsterdam reported postoperative glycemia as an independent risk factor for post-operative infections.12 Furthermore, Wengrovitz et al reported a significant association between diabetes and the onset of vascular surgical infections, likely associated to local immune deficiency and altered inflammatory response.13
Microbiology
Traditionally Staphylococcus aureus was described as the most frequent microorganism identified in VGI. However, coagulase-negative staphylococci have recently emerged as the main cause of VGI and account for over two thirds of all VGI. This is likely due to appropriate perioperative prophylaxis coupled with improved operative techniques and sterilization procedures.4,5,11,14,15 Other organisms that are normal indigenous flora, such as Corynebacterium spp., and Cutibacterium acnes, account for an increasing proportion of graft infections. In these cases, and contrary to staphylococcal infections, the infection tends to be delayed in onset.4 Enterococcal species have also been identified, often as part of polymicrobial infection along with anaerobic bacteria.11
Other factors that have contributed for changes in the microbiology of graft infections are changes in hospital flora, risk factors for nosocomial infections, such as multiple revisions of previous vascular surgery, and the presence of aortoenteric fistula (increasing the risk for polymicrobial infection due to gastrointestinal microbiota). Indeed, a more diverse spectrum of microbiota is often found in patients with VGI, including multidrug resistant strains (especially gram-negatives and methicillin-resistant Staphylococcus aureus - MRSA), polymicrobial infection and Candida spp.11 Indeed, Pseudomonas aeruginosa is the most common gram-negative, accounting for 10% of VGI.4,5 In Portugal, Gouveia e Melo found that in the cohort of a large university hospital, most intracavitary VGI were late infections most often due to Gram-negative bacteria (58%). Of the Gram-positive bacteria, most were coagulase-negative staphylococci or enterococci.7
In certain populations with specific risk factors, the suspicion for specific microorganisms such as Pasteurella multocida (in patients with animal scratches or bites), Salmonella spp. and Coxiella burnetti is relevant.5,16 Vascular Q fever, a well-recognized VGI in its own right, has been described as underlying some polymicrobial infections, perhaps increasing the risk for superinfection, and its recognition and treatment is paramount.16
Clinical presentation and diagnosis
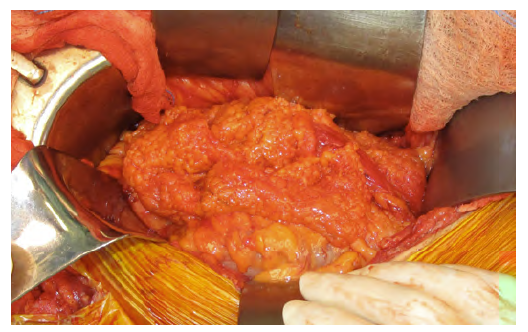
Especially in intracavitary infections, symptoms and clinical manifestations of graft infection may be subtle. Sepsis with no identified cause or prolonged ileus may be the only signs.9 All patients with a suspected intracavitary graft infection should be thoroughly tested with full blood counts, which may show leucocytosis and an increase in acute inflammation markers. Furthermore, blood cultures should be collected, as well abdominal fluid and urine cultures, to assess any adjacent infectious sources for a potential aortic graft infection.4,9 Since graft contact to adjacent bowel is a potential source of erosion and infection, vascular surgeons should be aware if the patient develops symptoms suggestive of an aortoenteric fistula with upper gastrointestinal bleeding.4,17 In this latter case, the use of endoscopic method is of great importance to detect cases of intracavitary graft infection.17 Besides endoscopic techniques, other imaging techniques such as CT scans or abdominal ultrasound are invaluable tools to detect early signs of graft infection, such as periprosthetic fluid or gas collections, wall thickening or false aneurysms, as well as fistulous paths (Figure 1).4,18,19
Figure 1 Typical finding of perigraft enhancement in a PET scan from a patient diagnosed with aortic graft infection (white arrows)
The role of PET scans is more relevant for late graft infections, as there are less obvious manifestations. Nevertheless, one must take into account some possible false-positives, such as other inflammatory states or surgery within the previous six months. In this case, it may help define and confirm the diagnosis of graft infection, as well as to assess the extent of the graft infection.18-20 A summary of the most frequent imaging exams used in aortic graft infection is provided in Table 1.
Treatment
Surgical management
As for any vascular graft infection, surgical strategies can be divided into graft-sparing techniques and graft explantation. The latter can be further distinguished into explantation with in situ reconstruction or extra-anatomical bypass.9,19 The choice of the appropriate approach depends on the surgeon’s experience, the extent of disease and the patient’s general status and comorbidities. A summary of the different surgical approaches on aortic graft infection is provided in Table 2.
Table 2 Summary on the different surgical approaches on aortic graft infection
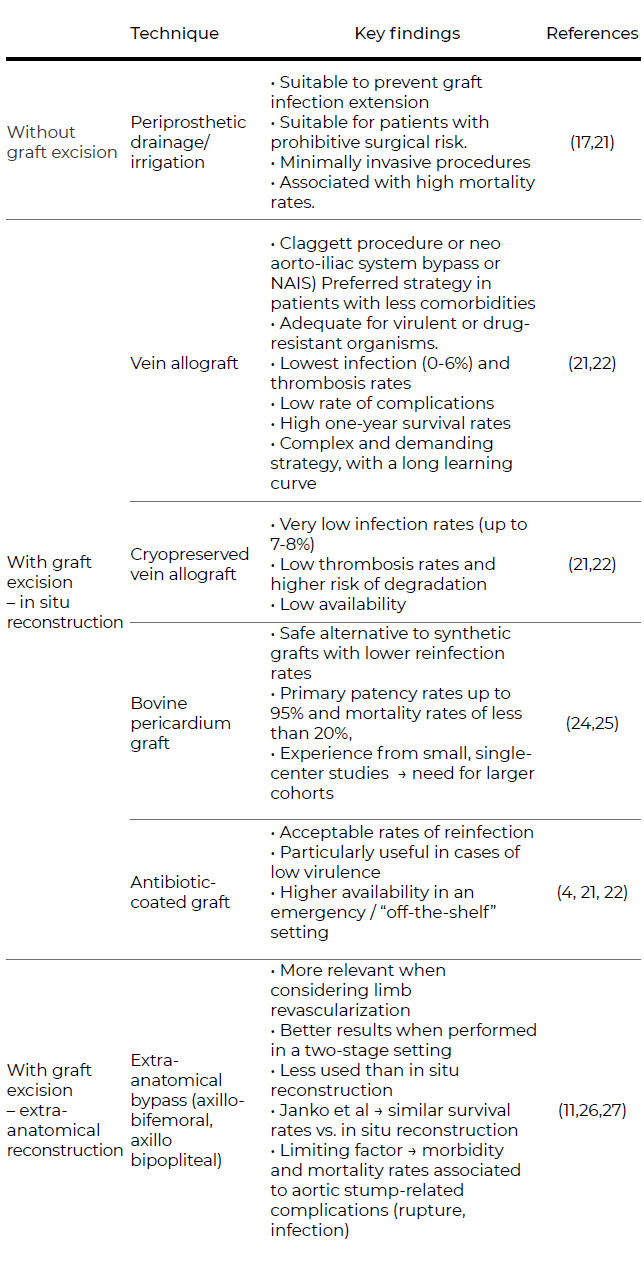
Note: aorto-enteric fistulas were excluded from this table as they require a specific treatment. Because of a more severe and complex presentation, there may be a need for more extensive resection and drainage, along with bowel resection and secondary reconstructions.
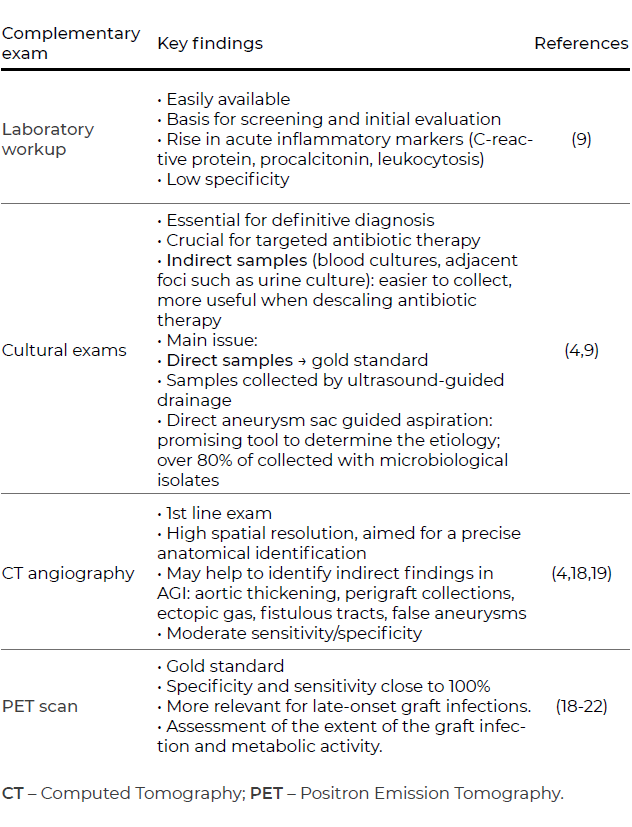
In the case of aortic graft infections, further strategies may be considered. More conservative approaches such as fluid drainage and irrigation are suitable adjunctive methods to prevent graft infection extension. However, these strategies are not a definitive solution and are associated with high mortality rates.17,21 When considering in situ reconstruction, antibiotic-coated grafts, such as those embedded in rifampin, are associated with lower rates of reinfection and are particularly useful in cases of low virulence and may be used in an emergency setting (Figure 2).
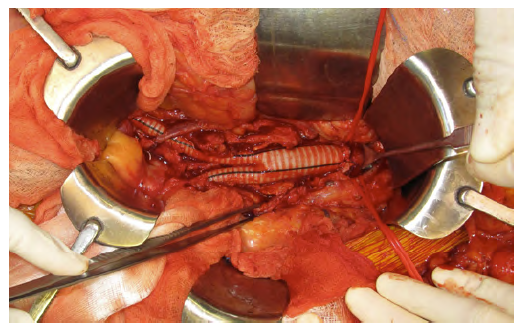
Figure 2A In situ reconstruction with antibiotic-coated Dacron graft after excision of an infected aortobifemoral bypass
This latter feature of rifampin-coated grafts is an advantage over cryopreserved allografts or the use of vein grafts, which may have the lowest rates of thrombosis and re-infection.4,9,21 This may be a preferred strategy in patients with less comorbidities or for AGI caused by more virulent or drug-resistant organisms.21,22 The use of deep femoral vein for abdominal aorta reconstructions (neo aorto-iliac system bypass or NAIS) was described by a group in Sweden as a surgical alternative after radical graft explantation or after removing aortic grafts in a bridge to surgery situation.23 Despite its feasibility and acceptable one-year survival rates (ten out of 12 patients), the authors recognize it remains a complex and demanding strategy. Furthermore, these imply an overall lower availability off-the-shelf. Lastly, bovine pericardium grafts represent a safe alternative to synthetic grafts with lower reinfection rates.24 Authors report primary patency rates up to 95% and mortality rates of less than 20%, close to the findings for other more widespread techniques.24,25 Although promising, these findings are derived from small, single-center studies, which urges the need for larger cohorts as well as longer follow-up periods.
In aortic graft infections, graft excision is crucial for an effective infection control. Regarding extra-anatomical bypasses (EAB), such as axillobifemoral bypasses, these are more relevant when considering limb revascularization after explanting the aortic graft.11,26,27 Although less used, a recent multi-center study from Janko and colleagues showed promising results. When comparing EAB with in situ repair, the latter does not seem to offer a significant survival benefit. Indeed, EAB patients did not have more complications or worse outcomes. The duration of antibiotic use was a significant factor for better surgical outcomes regardless of the surgical technique. Furthermore, Seeger et al showed satisfactory results in two-staged procedures.27 Patients with aorto-enteric fistula are particularly demanding to treat and intervene. In such cases, there may be a need for more extensive resection and drainage, along with bowel resection and secondary reconstructions. Because of a more dramatic and serious presentation, these patients are prone to higher rates of reintervention and postoperative complications, with mortality rates close to 100% in case of reinfection.19,21
Antimicrobial treatment
The selection of the antimicrobial regimen should be personalized and made in a multidisciplinary team including the vascular surgery team, an infectious diseases specialist, the microbiology department and sometimes a clinical pharmacist.4 Still, empiric parenteral antibiotic therapy should usually be promptly started, with targeted activity against the expected microorganisms. As such, the knowledge of local microbiological epidemiology is paramount. Additionally, the antibiotic regimen should be chosen considering its antibiofilm activity (therefore enabling it to penetrate the biofilm), and accounting for its pharmacokinetic profile and nonvascular complications (such as sepsis or metastatic involvement of other organs). Once the antimicrobial susceptibilities become available, antimicrobial therapy should be adjusted and/or deescalated to cover the relevant microorganisms.5,7 Initial therapy usually includes broad Gram-positive coverage (including an anti-MRSA drug) and broad Gram-negative coverage (including P. aeruginosa coverage), for example with piperacillin/tazobactam (or fluoroquinolones in patients with penicillin allergy) plus vancomycin, daptomycin or linezolid for MRSA coverage. Double-coverage of Gram-negative should be considered in settings of high probability of multidrug resistant Gram-negative bacilli. If according the local epidemiology, an antibiotic in monotherapy isn't expected to be active against >90% of the expected pathogen, a double coverage regiment (such as an association of betalactamic with an aminoglycoside) should be considered to appropriate therapy (defined as "at least one of the antimicrobial agents included in the chosen regiment has in vitro activity against the etiologic pathogens").5,28-30 Likewise, Gouveia e Melo reported that, in their Portuguese hospital, piperacillin/tazobactam with amikacin, plus an anti-MRSA drug is the better empiric therapy to chose especially if the patient presents severe disease or shock, due to the high prevelance of multidrug resistant gram-negatives in intracavitary VGI's (all extended-spectrum beta-lactamases producers Enterobacterals and multidrug-resistant non-fermenters).7
Conservative treatment with antimicrobial therapy alone, without surgical source control, carries a high mortality.31 Whilst there are no clinical trials evaluating the optimal duration of antimicrobial therapy in VGI, there is consensus that at least 4 to 6 weeks of parenteral therapy is appropriate. Afterwards, an additional 3 to 6 months of oral therapy should be considered and individualized for each patient, considering the microorganism recovered and possibly the presence of elevated biochemical markers of inflammation.4
An individualized approach in certain high-risk patients where surgery cannot be considered due to comorbidities, graft characteristics or extensive peri-graft infection, is possible. In these cases, lifelong suppressive antimicrobial therapy can be considered.4 In such cases, assessment through PET scans has been proposed as a potential means to guide the duration of antimicrobial therapy. Despite its more limited availability, this imaging modality might soon become a valuable tool in the diagnosis and management of patients with VGI. In some patients where lifelong therapy would be considered, the identification of a primary source of infection and only secondary infection of the vascular graft might be important, since in these cases antimicrobial therapy alone may be enough to eliminate the pathogen.18,32
Prognosis
Despite all efforts in developing adequate surgical approaches and life-long antibiotics, aortic graft infections still carry a significant burden in patients subjected to open aortic repair. Indeed, Vascular graft infections carry a high morbidity and mortality rate (24% - 80%) that can reach 100% within the first 2 years if the graft is left in situ, or there is a lack in prompt diagnosis and treatment of a primary or secondary aortoenteric or aortobronchial fistula.4,5,33-35 Nevertheless, Seeger et al, reported an early mortality rate of 19%, an amputation rate of less than 5%, early graft failure in 20% and a 58% survival rate without amputation. The mortality rate was highest among patients presenting with sepsis, aortoenteric fistula and those treated with simultaneous graft excision and extra-anatomic bypass.
Focus should therefore be shifted towards preventive measures. Firstly, preoperative antibiotic schemes may contribute to a lower rate of surgical revisions due to graft infection. In a retrospective study from Salmoukas and colleagues, preoperative antibiotic schemes with daptomycin contributed to lower rates of in vivo bacterial infection and abscess formation.36. As previously stated, the use of autologous tissue or antibiotic-bonded grafts may also mitigate the rate of this complication.
Conclusions
Vascular graft infections are a rare and severe complication of vascular surgery, particularly in its intracavitary form such as in patients submitted to open aortic repair. They can be severe and difficult to identify and recognize. A swift diagnosis and management with prompt source control and antimicrobial therapy is paramount. As such, this narrative review sought to thoroughly provide the most recent evidence on aortic graft infection, as there is a surge in simple and complex aortic interventions. Despite significant efforts on how to treat the infection with more adequate antibiotic and surgical therapies, effective preventive measures and identifying more susceptible populations remain the main areas for further developments. Indeed, there is a need for multi-center prospective studies to provide robust conclusions on who is truly at risk for this rare but dreadful complication.