Background
Secondary sclerosing cholangitis (SSC) is a severe disease characterized by progressive injury to bile ducts inflicted by autoimmune mechanisms, infections, drugs, ischemia, or obstruction [1]. SSC in critically ill patients (SSC-CIP) is a rare form of SSC first described in 2001 [2]. It is frequently underdiagnosed, since half of the patients die before a diagnosis can be made [1].
SSC-CIP manifests as cholestasis in critically ill patients with no prior history of hepatobiliary disease and no known pathologic process of injury responsible for bile duct obstruction [3]. The feature that distinguishes this entity in the intensive care unit (ICU) setting is the persistence of cholestasis beyond clinical recovery, reflecting irreversible anatomical damage. As such, prognosis is poor, and patients end up developing acute liver failure during ICU stay or progressive cholestasis rapidly progressing to biliary cirrhosis [1].
There has been a greater awareness of this underdiagnosed condition in the last years, reflected in the number of published literature case reports. We present a case of SSC-CIP precipitated by a severe SARS-CoV-2 infection during the current global pandemic, thereby creating an excellent learning opportunity about these two entities and promoting SSC-CIP early recognition in the critically ill patient.
Case Presentation
A previously healthy 46-year-old woman was admitted to the ICU with a 1-week history of progressively worse cough and breathlessness in the context of known SARS-CoV-2 infection. She had a medical history of hypertension and class III obesity (BMI 44 kg/m2). Admission blood tests revealed lymphopenia 920/L, d-dimers >32.50 mg/L, ferritin 2,371 μg/L, C-reactive protein 15.72 mg/dL, procalcitonin 0.87 ng/mL, troponin 2.08 μg/L, aspartate aminotransferase (AST) 54 UI/L, and lactate dehydrogenase 797 UI/L. The remaining liver function tests (LFTs) were normal. Chest computed tomography suggested SARS-CoV2 pneumonia with extensive lung involvement. Due to severe and refractory hypoxemia, the patient was sedated with propofol and ketamine and subsequently intubated and ventilated on the same day of admission. She was treated with dexamethasone and anticoagulated with enoxaparin.
During ICU stay, she had a period of hypotension that required vasopressor support for a period of less than 24 h. She was treated with a course of piperacillintazobactam that was later deescalated to amoxicillin-clavulanate for ventilator-associated pneumonia and bacteremia to Klebsiella pneumoniae. Prone ventilation was performed in four occasions and low volumes with high positive end-expiratory pressure were used. After weaning from sedation, she was successfully extubated after 12 days.
While in ICU, it was noted that the patient’s LFTs were becoming deranged with a predominantly cholestatic pattern. Prothrombin time remained within normal range (Fig. 1).
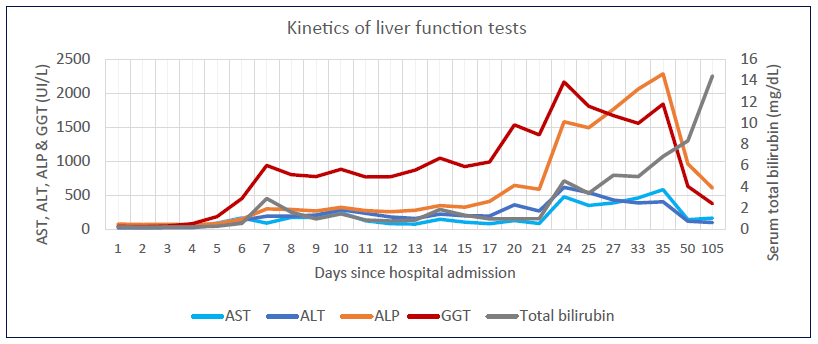
Fig. 1. Kinetics of liver function tests since hospital admission. AST, aspartate aminotransferase; ALT alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase.
The patient was discharged to the medical ward after clinical improvement. She was further treated with meropenem due to nosocomial urinary tract infection due to multidrug-resistant Klebsiella pneumoniae. Blood and urine cultures were later repeated and were negative. However, blood tests revealed continuous worsening of LFTs.
Investigations
An abdominal ultrasonography was performed after ICU discharge and revealed liver steatosis and hypoechoic hepatic nodules suggestive of non-pure cystic nature, suspicious for hepatic abscesses.
Abdominal computed tomography and magnetic resonance imaging with cholangiopancreatography (MRCP) revealed several nodules in the liver suggestive of abscesses and mild dilation of the intrahepatic bile ducts with multiple strictures and beaded appearance, suggestive of sclerosing cholangitis. At the bifurcation of the common hepatic duct (CHD), there was a linear and trilaminar repletion defect, raising the suspicion of sludge or parasite (Fig. 2). There was no involvement of the common bile duct.
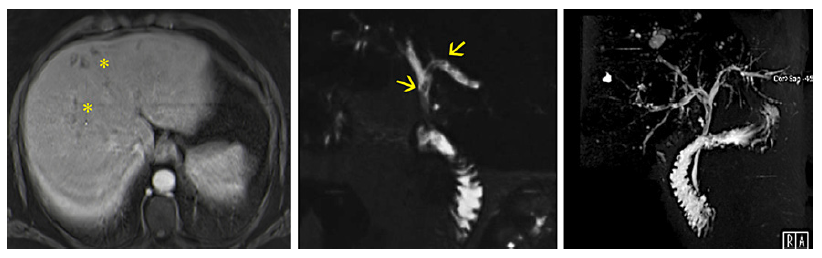
Fig. 2. Magnetic resonance imaging with cholangiopancreatography. Left - T1 portal venous phase with subcapsular clustered hypointense lesions with peripheral enhancement (*). These lesions had a hyperintense signal in the T2 sequence, suspected of microabscesses. Middle - cholangiographic T2 sequence, showing linear repletion defects at the bifurcation of the common hepatic duct (arrows). Right - cholangiographic 3D sequence showing dilation of intrahepatic bile ducts, with parietal irregularity, strictures, and post-stenotic dilations, suggesting sclerosing cholangitis.
An endoscopic retrograde cholangiopancreatography (ERCP) was performed, revealing irregular intrahepatic bile ducts along with an ill-defined lacunar image at the bifurcation of the CHD (Fig. 3).
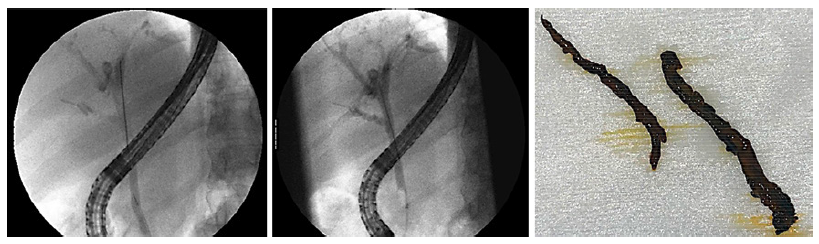
Fig. 3. Endoscopic retrograde cholangiopancreatography. Left - ERCP X-ray images after injection of contrast media, showing an ill-defined filling defect at the common hepatic duct bifurcation and irregular filling of intra-hepatic bile ducts. Right - biliary casts that were retrieved from the common hepatic duct and left and right hepatic ducts, using an extraction balloon and a Dormia basket.
Serologies for human immunodeficiency virus, hepatitis B and C virus were negative. A panel of autoimmunity (including antinuclear, antimitochondrial, anti-smooth muscle, anti-liver-kidney microsomal and anti-neutrophilic cytoplasmic autoantibodies), immunoglobulin levels (including IgG-4 subclass), α1-antitrypsin and serum caeruloplasmin levels was within the normal range. The 24-h urinary copper was high, indicating cholestasis. Ferritin levels peaked at 11,924 μg/L, but transferrin saturation remained within normal values.
Serologies for Epstein-Barr, cytomegalovirus, Entamoeba histolytica, Echinococcus granulosus and Fasciola hepatica were negative.
Differential Diagnosis
During ICU stay, altered LFTs were thought to be the result of a combination of pathophysiological factors including SARS-CoV-2-induced systemic inflammatory response injury and drug-induced liver injury (DILI) associated with anesthetics and antibiotics. A conservative approach was taken at this point. However, despite respiratory insufficiency recovering, LFTs continued to deteriorate, and a pronounced worsening with a predomi-nantly cholestatic pattern was seen 3 days after ICU discharge. There was no history of prior hepatobiliary disease that could have been decompensated by the current SARS-CoV-2 infection. DILI associated with anesthetics or antibiotics was a potential cause; however, it could not explain the magnitude of the cholestatic pattern and the lack of improvement upon discontinuation of potential causative drugs. Imaging played a decisive role, since MRCP revealed the presence of liver abscesses and irregularities in the intrahepatic bile ducts with beaded appearance, while excluding dilation of the main bile duct. Looking at the whole picture, a diagnosis of SSC-CIP was strongly suspected and later confirmed during ERCP after biliary cast removal.
Treatment
Patient was kept on meropenem once the radiological exams revealed liver abscesses; blood cultures were repeated on three occasions and were all negative, so no adjustments were made.
During ERCP, sphincterotomy was performed and biliary casts were retrieved from the left and right hepatic ducts using the extraction balloon and Dormia basket (Fig. 3). Histopathologic analysis supported the finding of biliary casts.
Outcome and Follow-Up
The patient was discharged 2 days after ERCP, after clinical improvement. Antibiotic therapy was continued with amoxicillinclavulanate for another week, and the patient was started on ursodeoxycholic acid (UDCA).
Nine months after hospital discharge, LFTs revealed a decrease in cytocholestasis, while total bilirubin continues to rise (24 mg/dL). The patient has jaundice and pruritus that is amenable to medical therapy. MRCP was repeated at 3 and 8 months after discharge: there was complete resolution of liver abscesses and persistence of irregularity of intrahepatic bile ducts compatible with sclerosing cholangitis; however, signs of impending liver cirrhosis ensue with hepatosplenomegaly and caudate lobe hypertrophy. The patient is currently referred to the regional liver transplant center.
Discussion
SSC-CIP is a rare form of SSC first described in 2001 [2]. Since then, 250 cases have been reported and most of them in the last 6 years, reflecting increasing awareness of this condition [1].
The diagnosis of SSC-CIP is made when a cause for progressive bile duct damage and obstruction is identified in a critically ill patient admitted to the ICU with no prior history of hepatobiliary disease. While most patients with SARS-Cov-2 infection develop mild-to-moderate disease, our patient became critically ill and developed a systemic inflammatory response syndrome (SIRS), respiratory failure and acute respiratory distress syndrome (ARDS). These features are suggestive of a cytokine storm syndrome, in which hyperinflammation and multiorgan disease can arise through excessive cytokine release from uncontrolled immune activation [4].
Two major concepts are thought to be the underlying pathophysiological mechanisms of SSC-CIP: the “ischemic cholangiopathy” and the “toxic bile.”
Ischemic Cholangiopathy
The biliary epithelium is prone to ischemic injury as a result of the blood irrigation supplied exclusively by branches of the hepatic artery, originating the peribiliary plexus. As such, disturbances of blood supply either at the level of the macro-circulation or microcirculation can trigger ischemic necrosis [5].
Hemodynamic instability with a decrease in mean arterial pressure <65 mm Hg was reported in 60-100% of patients with SSC-CIP [1], with a temporal relation between the onset of severe hypotension and development of cholestasis [5]. Epinephrine and norepinephrine frequently used in this context have a further dose-dependent negative effect on the perfusion of visceral organs [5]. Contrary to what was previously thought, even low doses of catecholamines and duration of catecholamines use or mechanical ventilation as short as only one day can trigger the development of SSC-CIP [5, 6].
Mechanical ventilation was reported in all patients with SSC-CIP [1]. High positive end-expiratory pressure (>10 cm H2O), low tidal volumes and prone position have demonstrated negative effects on the microcirculation of the gastrointestinal tract in animal models [1, 5]. This strategy of ventilation was adopted in our patient since it is the one recommended to treat the SARS-CoV-2 associated ARDS [7]. Furthermore, obesity and prone ventilation appear to increase the risk of SSC-CIP in influenza A associated ARDS [8]. Therefore, both mechanical ventilation strategy and obesity have probably played a role for SSC-CIP development in our patient. Increased blood viscosity and hypercoagulable states can also compromise the microcirculation in the peribiliary plexus [5]. SIRS is a major contributor to SARS-Cov-2 associated coagulopathy, supporting the concept of thromboinflammation [9]. Such hypercoagulable state was clearly evident in our case (through a marked elevation in d-dimers), and that could also have contributed to biliary ischemic injury [5].
Toxic Bile
The lipid cellular membrane of cholangiocytes is protected from the detergent properties of hydrophobic bile acids by the hepatocellular secretion of phospholipids and the biliary secretion of HCO3- [5]. These cellular transport mechanisms can be compromised by proinflammatory cytokines, released in several clinical conditions such as SIRS/sepsis, trauma, burns, and major surgery [5]. Our patient was admitted with signs of impending hyperinflammation such as lymphopenia, elevated d-dimer, tissue damage/hepatitis (elevated lactate dehydrogenase, AST, and alanine aminotransferase), and macrophage/hepatocyte activation (elevated ferritin) [4]. Such SARS-CoV-2 induced SIRS, together with the period of severe hypotension (that can further induce SIRS via tissue ischemia), have probably contributed to the disruption of these protective mechanisms. Ischemia can also directly downregulate hepatobiliary transporters [5].
Bile duct damage in SSC-CIP can be a very early event, manifesting within the first 5 days of ICU admission through a rise in cholestatic enzymes [10]. Gamma-glutamyl transpeptidase is the first enzyme to become elevated peaking at 20-50 times the upper limit of normal (ULN), followed by alkaline phosphatase peaking at 5-21 times the ULN and later by bilirubin; AST and alanine aminotransferase can be moderately elevated (up to 20 and 9 times the ULN, respectively, in one case series) [10]. This pattern of abnormal LFTs was seen in our patient (Fig. 1). Of note, liver impairment has been reported in up to 53% of patients with SARS-CoV-2 infection reflecting hepatic involvement by a severe systemic inflammatory disease, although direct viral injury to hepatocytes cannot be excluded [11, 12]. However, SARS-CoV-2-as-sociated LFT elevation occur predominantly in a hepato-cellular pattern [11, 13, 14].
The differential diagnosis for cholestasis in the ICU setting is extensive, including sepsis, total parenteral nutrition, choledocholithiasis/cholangitis, DILI, and hypoxic liver injury [1]. A final diagnosis of SSC-CIP can only be established by imaging. Typical MRCP findings include filling defects in the intrahepatic biliary tree with diffuse strictures with beaded appearance appearing in later stages. ERCP remains the gold-standard method, allowing for biliary casts removal [1]. Importantly, the distal common bile duct is preserved in SSC-CIP, due to its dual blood supply (originating from the hepatic artery and gastroduodenal artery), differentiating it from other potential causes of SSC. Furthermore, biliary casts are ex-clusively seen in SSC-CIP and ischemia-induced SSC. These two entities can be distinguished from each other by the presence of extrahepatic bile duct involvement and a predominantly hepatocellular pattern in LFTs, which are only seen in ischemia-induced SSC [1].
SSC-CIP has a dismal prognosis, with mortality rates as high as 50% during ICU stay [1]. In a reported series, 38% of patients who survived rapidly progressed to cir-rhosis in 18 months [15].
Antibiotic and endoscopic therapy with endoscopic dilation, sphincterotomy and sludge extraction were per-formed in most reported patients [15]. The addition of UDCA to endoscopic therapy seems to contribute to LFT normalization [16]. Once cirrhosis develops, orthotopic liver transplant (OLT) is the only curative treatment and outcomes seem comparable to other indications of OLT, with 1-, 3-, and 5-year survival rate of 85-100%, 83-86%, and 76%, respectively [10].
Learning Points
SSC-CIP is a rare form of SSC that must be considered in critically ill patients with no previous history of hepatobiliary disease that develop persistent cholestasis
Hemodynamic instability, mechanical ventilation and SIRS are among the most important triggers for its development
Severe SARS-CoV-2 infection alone can cause a de-rangement in LFTs in a predominantly hepatocellular pattern and tend to follow the disease course
Typical findings in MRCP are required for the diagno-sis of SSC-CIP, including the presence of filling defects in the intrahepatic biliary tree and diffuse strictures with beaded appearance in later stages;
Removal of biliary casts during ERCP further supports the diagnosis of SSC-CIP, although they can also be seen in ischemia-induced SSC (which can be distinguished by the involvement of extrahepatic bile ducts and by a predominantly hepatocellular pattern in LFTs).















