Introduction
Dermatologists have at their disposal numerous immunosuppressive drugs that are useful in controlling several conditions such as autoimmune bullous dermatoses, psoriasis, and connective tissue disease1,2. Indeed, it is imperative that physicians are aware of the iatrogenic increased risk and severity of infection. As such, screening for several latent microorganisms can be valuable (e.g., tuberculosis, hepatitis B and C, deep fungal infections, or HIV)1,2.
One such parasite is Strongyloides stercoralis. It is a skin-penetrating intestinal nematode with a complex life cycle3. It is widely distributed around the world especially around the tropics3 and is one of the few helminths with the ability of autoinfection4. Since it can present as a hyperinfection syndrome that occurs especially among patients with immunosuppressive conditions or therapies4–6, it is relevant to refresh its epidemiology and pathology, with a focus on the role of its screening prior to the start of immunosuppressive therapy.
Epidemiology
Strongyloidiasis is an emerging infection with a worldwide incidence underestimated in many countries7. It has an estimated global prevalence of over 350 million people8. While it has been traditionally described among patients from tropical and subtropical countries3,9, the prevalence of infection has been increasing not only in Caribbean, Southeast Asia, Latin America, and sub-Saharan Africa, but also in southern, eastern, and central Europe6,9. While Portugal is currently considered a nonendemic country, with infection with S. stercoralis being found especially among immigrants from endemic countries, the prevalence of strongyloidiasis and other helminthiasis was higher during the first three quarters of the 20th century, likely due to a lack of basic sanitation conditions10. During this time, S. stercoralis was found especially in the regions between the Douro and Tagus rivers10, and even very recently, a case of a Portuguese woman presenting with strongyloidiasis was reported9, even though likely infected long ago11. Moreover, in 2001, in a cohort of children from Lisbon, 0.9% were found to be infected12.
In many endemic areas, where moist soil, temperate or tropical climate and improper disposal of human waste coexist, the prevalence of strongyloidiasis can reach 50%. This is especially the case in West Africa, the Caribbean, Southeast Asia, Brazil, Cambodia, and some regions of Spain. Nevertheless, Southeast Asia seems to have the highest endemic prevalence13,14. Other risk factors for infection include white males, working with soil and travellers to areas of endemicity6. Although strongyloidiasis occurs in all ages, infection usually happens in childhood, since children are more likely to play outdoors with higher exposure to contaminated soil12,15.
Patients with certain immunosuppressive conditions are also at a higher risk for strongyloidiasis. Indeed, an altered cellular immunity (especially those on long-term corticosteroid therapy, but also human immunodeficiency virus [HIV] infection/acquired immunodeficiency syndrome), certain hematological malignancies and therapies (such as those for lymphoma and allograft transplant recipients) are at a higher risk for severe strongyloidiasis infection5,16,17. Human T-lymphotropic virus type 1 (HTLV-1) infection is also related to S. stercoralis with increased prevalence of this parasite in overlapping endemicity areas3,18. Indeed, corticosteroid treatment and HTLV-1 infection are the two conditions most associated with hyperinfection16.
Lifecycle and transmission
Strongyloides stercoralis has a complex life cycle with two unique and distinct cycles (Fig. 1). While transmission usually occurs through contact with contaminated soil, person-to-person transmission has been described, especially among men who have sex with men19.
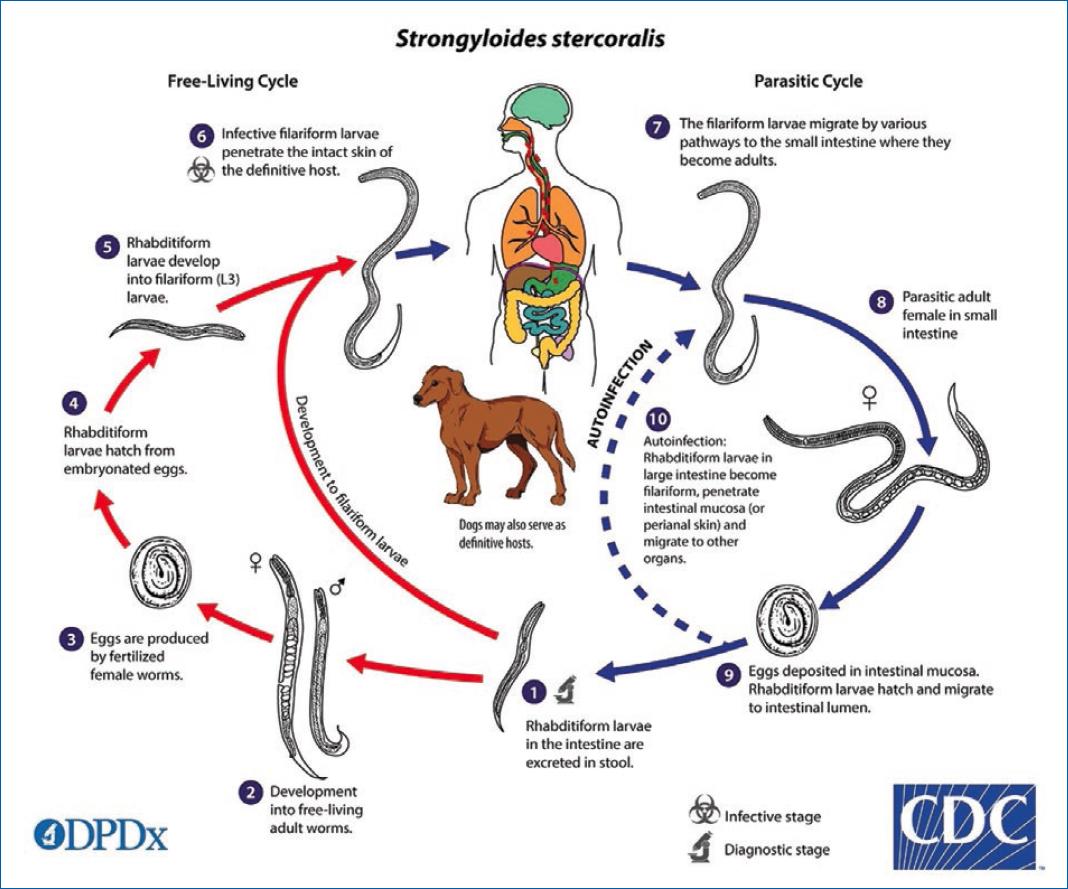
Figure 1 Strongyloides stercoralis life cycle (Image from courtesy of DPDx, a website by the Centers for Disease Control and Prevention (CDC)’s Division of Global Health, Parasitic Disease and Malaria)54. It consists of a free-living cycle in the soil, where both males and females coexist and maintain infestation in the ground. Here, eggs are hatched as rhabditiform larvae and afterward transformed into infective filariform larvae. In this stage, the larvae penetrate the skin and migrate to the small intestine where they mature into adult females and produce eggs parthenogenetically. These hatch into rhabditiform larvae that are excreted in the stool and can lead to autoinfection. These parasitic females may live up to five years, continuing their reproductive cycle6,18,20.
As one of the few helminths that is able to autoinfect its human host20, rhabditiform larvae can fertilize into its filariform stage in the large bowel. Afterwards they migrate through the lymphatic and venous circulation, reaching the pulmonary circulation, alveolar space, and crawling up the respiratory tract. Then they return to the intestine through swallowed sputum6,18. External auto-infection can also occur, in which case it often leads to the development of larva currens6.
Almost all strongyloidiasis are due to infection with Strongyloides stercoralis. However, the primate parasite Strongyloides fulleborni has been described in children in Africa and in Papua New Guinea, where it is a cause of “swollen belly syndrome”20.
Pathogenesis and clinical manifestations
Strongyloides stercoralis infection was first described in 1876 from the stool of French soldiers with diarrhea who were returning from the old Indochina region, leading to the designation of “Cochin-China diarrhoea”21. Manifestations of primary acute infection with Strongyloides stercoralis are directly related to its life cycle. After skin penetration, if the larvae do not find their natural route to the circulation and stay in the integument, larva migrans presenting as a maculopapular, pruriginous and serpiginous rash can occur22.
Manifestations of acute and chronic strongyloidiasis can be found in Table 1. Chronic infection is often asymptomatic, with eosinophilia being the sole, albeit intermittent, marker9,23. Actually, hypersensitivity is an important part of the immune response to this parasite, contributing both to the pathogenesis of the disease and to its protection16. In fact, a primary Th2 response favors infection by increasing tissue permeability to the parasite and reducing complement activation, important for the larvae-killing capabilities of eosinophils and granulocytes24,25, but interleukin-13 causes increased peristalsis, possibly leading to increased larval expulsion25. On the other hand, HTLV-1 infection, a known risk factor for severe strongyloidiasis, results in an increased interferon-gamma production and decreased levels of interleukin-4 and IgE, which creates a favorable environment for Strongyloides stercoralis proliferation16.
Table 1 Principal manifestations of acute and chronic strongyloidiasis7,9,20,22,23,26,55. Strongyloidiasis presentation directly relates to the parasite’s life cycle. Acute infection can be asymptomatic in up to one-third of infections. Chronic strongyloidiasis is often asymptomatic, with eosinophilia being the sole, albeit intermittent, marker. Otherwise, gastrointestinal symptoms can occur, with larva currens, abdominal pain, and diarrhea being a classically recognized triad
| Acute strongyloidiasis | Chronic strongyloidiasis | |
|---|---|---|
| Gastrointestinal manifestations | Abdominal pain, malabsorption, steatorrhea, diarrhea Onset usually 2 weeks after infection; larvae found on the stool after 3-4 weeks |
Diarrhea, malabsorption, steatorrhea, constipation, abdominal pain, intermittent vomiting |
| Respiratory manifestations | Cough, wheezing, shortness of breath, tracheal irritation, bronchitis, Loeffler’s syndrome, transient pulmonary infiltrates Onset a few days after infection |
Cough, dyspnoea, recurrent asthma Often mild or absent |
| Other | Larva migrans, fever, anorexia Eosinophilia (as high as 75-80%) |
Intermittent eosinophilia and elevated IgE levels, often isolated Nephrotic syndrome Pruritus ani, larva currens, urticarial, petechial and purpuric rashes |
Whereas internal autoinfection is usually less relevant in healthy individuals, in immunosuppressed patients it can present as one the two most severe forms of strongyloidiasis, either the hyperinfection syndrome or the disseminated disease. Although immunocompetent patients are also at risk, those with impaired cell-mediated immunity are much more susceptible6,26. In severe strongyloidiasis in the immunocompromised host, eosinophilia is often absent23. In the hyperinfection syndrome there is a favorable environment for parasitic proliferation, resulting in an increased burden along the usual migration pattern. It essentially is an accelerated auto-infection and the distinction between these two is merely quantitative and not strictly defined26.
As such, new onset or exacerbation of gastrointestinal and pulmonary symptoms is frequent, and the identification of increased numbers of larvae in faeces and/or respiratory samples is the hallmark of hyperinfection26. While the increased numbers can lead to complications such as intestinal obstruction, ileus, and gastrointestinal bleeding, usually there is no metastatic dissemination outside the regular migration pattern. Nevertheless, migration of larvae that carry bacteria on the surface of the larval integument, as excreta from the larval intestinal tract27 or the presence of ulcers may facilitate the spread and systemic infection with enteric bacteria26.
Pulmonary complications including pulmonary infiltrates, diffuse alveolar hemorrhage, and respiratory failure can develop in patients with hyperinfection syndrome and, if not treated, may be lethal. Indeed, a lack of familiarity with this parasite leading to delayed screening and treatment is a cause for a high mortality among immunosuppressed patients28.
While hyperinfection denotes an increased parasite replication, disseminated strongyloidiasis implies widespread dissemination to extraintestinal organs, without the obligatory need for an increased parasite proliferation or severity of disease26. Multiple organs beyond the range of its normal life cycle are affected, including the liver, heart, kidneys, and central nervous system6. In severe disease, and as in hyperinfection, translocation of enteric bacteria can occur, leading to polymicrobial bacteriemia or meningitis6. Cerebrospinal fluid analysis shows neutrophilic pleocytosis with an elevated protein level and low glucose level. A gram stain can be positive for enteric bacteria and direct examination can reveal Strongyloides stercoralis larvae29,30.
Other manifestations include lymphadenopathy, fever, haemoptysis, cough, anaemia, vomiting, weight loss, abdominal pain, and distension31. Since it coexists frequently with hyperinfection syndrome in the immunosuppressed patient, its manifestations may overlap6.
Diagnosis
Since most patients with strongyloidiasis do not present with distinct clinical features, the diagnosis requires a high degree of suspicion11.
Strongyloides stercoralis larvae can be intermittently found in faeces usually a month after skin penetration. Usually, only larvae are found since the eggs immediately hatch in the intestine. Strongyloides fulleborni, however, sheds eggs in faeces, and is readily found using microscopy6. Direct smear examination of stool in saline and Lugol’s iodine stain is a definitive diagnostic testing, although with a low sensitivity (as low as 21%). However, concentration methods, such as formalin-ethyl acetate, Harada-Mori techniques, and Baermann concentration increase the yield and are significantly more sensitive32,33. While diagnosis of hyperinfection is relatively easy due to the high quantity of larvae in stool and sputum, outside of this setting it is often inadequate, as a single stool examination is less than 50% sensitive for making diagnosis34. As such, it is mandatory to screen multiple times, ideally using a concentration method, although they are seldom performed in most parasitology labs34,35. A sensitivity higher than 90% can be achieved if seven or more samples are examined35. When concerning the hyperinfection syndrome, the examination of a duodenal aspirate for eggs and larva is the most sensitive diagnostic procedure (as high as 90%)31.
In addition to faeces samples, endoscopic examination and biopsies can be useful. Endoscopy may range from normal-appearing mucosa to severe duodenitis or colitis with oedematous and erythematous mucosa and white villi. Moreover, in hyperinfection with pulmonary involvement, larvae can be shown in duodenal biopsy36. In disseminated disease, larvae can be found in several extraintestinal sites, such as skin biopsy, cerebrospinal fluid, urine, pericardial, pleural and peritoneal fluid37–42.
Serological assays are another useful tool in the diagnosis and follow-up of strongyloidiasis. Specific antibodies can be used as a follow-up to prove seroconversion after a successful therapy. There are several commercially available tests with varying sensitivities and specificities. For example, ELISA seems to be a sensitive test (88–95%), albeit with a variable specificity (29–99%)34. The low specificity is due to cross-reactivity with other helminth infections, such as filariasis, ascariasis and acute schistosomiasis34. However, in Portugal, these are likely not frequent differential diagnosis, and this appear to be a smaller issue with more recent test kits43. Another drawback of these tests is their lower sensitivity in severely immunosuppressed patients, and incapacity to accurately distinguish between past and present infection among patients already treated for strongyloidiasis or originating from an endemic country44. However, antibody titres tend to diminish with time, although the time required to become negative may be higher than 12 months5.
Real-time polymerase chain reaction is another tool for the diagnosis of strongyloidiasis, albeit not being readily available in most centres. Estimates of sensitivity of this method are variable but seem high. In the future, molecular testing may enhance the diagnosis of this infection6,34.
Screening and management
Strongyloidiasis should be a differential diagnosis in any patient with unexplained eosinophilia, especially if there was exposure in endemic areas. However, immunocompetent patients with high risk of exposure should still be screened, even if without eosinophilia5. Moreover, in patients with risk factors for developing hyperinfection, testing should also be considered, particularly when having a history of originating or travelling to an endemic country, even if in a distant past5,11,25. This is especially important in patients that have immunosuppressive conditions or treatments, such as those with hematologic malignancies, undergoing transplantation or corticosteroid therapy5. Indeed, in this case, both parasitological and serological assays should be used5,25. In some cases, pre-emptive ivermectin treatment should be considered, if a diagnostic test is not available5. However, although corticosteroid exposure has been identified as the main risk factor, there are also reports regarding the use of non-steroid immunosuppressive agents and biologic therapies, including those directed at IL-1, TNFα and lymphocyte depleting drugs45,46. Nevertheless, while IgE, IL-13, and IL-4 are paramount for the pathogenesis of this disease, unexpectedly, the modulation of these cytokines has not yet been found to increase risk of strongyloidiasis. Still, pre-treatment screening is advised47,48. Additionally, screening should also be considered in those with evidence of HTLV-1 infection49.
Dermatologists can take advantage of numerous immunosuppressive drugs in the management of several ailments such as chronic immunoinflammatory diseases, psoriasis, and connective tissue disease. It is, therefore, imperative that a screening for the relevant opportunistic diseases be considered prior to the start of treatment. Strongyloidiasis is one such illness, and several guidelines recommend its screening prior to the start of several medications, primarily corticosteroids1,2,50. To do so, likely a combination of serological and microbiological assays is ideal, since in general, serology is highly sensitive, while stool examination is highly specific. Moreover, immunosuppressed patients may have a lower serological sensitivity which might be overcome by an increased detection in stool samples45,51. As such, a pre-treatment screen with several (perhaps more than seven) stool samples and serology is advised.
Treatment of strongyloidiasis is usually performed with ivermectin. This broad-spectrum antiparasitic causes muscle paralysis in invertebrates by activating chlorine channels52. It is better tolerated and has a similar efficacy than thiabendazole, and is more effective than albendazol5,25. In uncomplicated infections a single 200 μg/kg/day oral dose of ivermectin for one or two days usually sufficient5. A repetition of this course could be suggested after two weeks, to account for the parasite’s autoinfective cycle, however a randomized clinical trial failed to show advantage in this strategy53.
When considering hyperinfection, there is a lack in high-quality evidence. However, it has been suggested that ivermectin should be given daily or every 48 hours at a dose of 200 μg/kg/day for at least one to two weeks. When the oral route is not well tolerated, alternative routes can be considered. Multiple follow-up stool assessments should be performed, and treatment continuation until no more larvae are found in faeces should be considered7,25. Additionally, whenever iatrogenic immunosuppression is present, reduction in these regimens should be considered, when clinically feasible25. Moreover, these patients should be considered infectious and put under standard contact precautions49.
The high mortality in hyperinfection is often due to a lack of awareness in the need for parasite screening before the start of corticosteroid therapy28. Indeed, several patients with fatal outcomes after treatment with empirical corticosteroids are later confirmed as a case of disseminated strongyloidiasis. Moreover, the possibility of infection with this nematode should be considered in any immunocompromised patient who suddenly deteriorates without any apparent cause, since delay in treatment often results in death14.
Conclusion
Currently, strongyloidiasis is a rare disease in Portugal, mostly related to migrant population. However, it can have a severe if not fatal course, especially amongst immunosuppressed patients. As such, and since chronic infection can often be asymptomatic, screening prior to the start of immunosuppressive treatment (especially corticosteroids) is imperative. Dermatologists that prescribe such regimens should be familiar with the need of parasite screening and management prior to the start of therapy.














